Abstract
Objective
Nurses are increasingly becoming involved in integrative oncology (IO) programs. This study examined the additive effect of nurse-provided guidance for self-administered IO therapies on cancer-related fatigue and quality of life (QoL).
Methods
The study was randomized and controlled, enrolling patients undergoing active oncology treatment with IO interventions for fatigue and other QoL-related outcomes. IO practitioner guidance on self-treatment with manual, relaxation, and/or traditional herbal therapies was provided to patients in both the intervention and control arms. However, patients in the intervention arms also received additional guidance on self-treatment by IO-trained palliative care nurses. All participants were assessed for fatigue and QoL at baseline and at 24-h follow-up, using the Edmonton Symptom Assessment Scale (ESAS) and the Measure Yourself Concerns and Wellbeing (MYCAW) questionnaire tools.
Results
Of 353 patients recruited, 187 were randomized to the intervention and 166 to the control group. Both groups had similar demographic and oncology-related characteristics. Patients in the intervention arm reported significantly greater improvement in ESAS scores for fatigue (p = 0.026) and appetite (p = 0.003) when compared to controls.
Conclusion
The addition of nurse-provided guidance on self-administration of IO treatments to that provided by IO practitioners further reduced short-term scores for fatigue and improved appetite. The relationship between palliative and IO-supportive cancer care requires further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The integration of complementary medicine in conventional cancer care has become increasingly prevalent in many oncology centers throughout North America and internationally, with the goal of improving the quality of life (QoL) in patients with evidence-based, effective, and safe treatments [1]. This approach, termed “integrative oncology” (IO), has been defined as “…a patient-centered, evidence-informed field of cancer care that utilizes mind and body practices, natural products, and /or lifestyle modifications from different traditions alongside conventional cancer treatments. Integrative oncology aims to optimize health, quality of life, and clinical outcomes across the cancer care continuum, and to empower people to prevent cancer and become active participants before, during and after cancer treatment” [2]. As the research on IO has expanded, clinical practice guidelines are being developed by the Society for Integrative Oncology (SIO) and subsequently endorsed by the American Society for Clinical Oncology [3].
By its very nature, IO adopts a multidisciplinary approach, engaging a diverse team of healthcare professionals, including oncology nurses with specialized training in IO. These nurses play an important role in designing oncology and palliative care training programs; conducting randomized controlled trials; and developing clinical practice guidelines [4, 5]. As part of the multidisciplinary IO healthcare team, the scope of practice among nurses includes providing information to patients and family members about complementary and integrative (vs. alternative) therapies [6]; referring patients to IO consultations [7]; and participating in the provision of IO treatments. Working together with the multidisciplinary palliative cancer care team, nurses trained in IO can address a wide range of QoL-related concerns such as pain, fatigue, anxiety, insomnia, and gastrointestinal symptoms [8].
Despite the central role nurses play as members of the supportive IO team, the research on their role and impact on patient outcomes has been limited. An exception is the Complementary Nursing in Gynecologic Oncology Study, which randomized 151 women with breast or gynecological cancer to either standard supportive cancer care, or supportive care with additional symptom management counseling and a select regimen of complementary therapies provided by specially trained nurses [8]. Significant differences in QoL were observed at 6 months following the completion of the intervention.
In order to promote the inclusion of nurses working in oncology, primary care, and hospice clinical settings in Israel in multidisciplinary IO teams, an IO training program was created to provide nurses with skills in complementary and integrative medicine, as well as palliative care [4, 9]. The present study examined the impact of nurse-provided guidance in the self-administration of IO therapies by oncology patients, as part of a comprehensive IO treatment regimen focused on reducing fatigue and improving QoL. It was hypothesized that the additional guidance and support provided by nurses trained in IO would decrease fatigue, other cancer-related concerns, and general well-being.
Methods
Study design
The study utilized a randomized controlled trial design. In this study, the term “palliative care” was based on the World Health Organization’s definition, in which supportive care is provided to patients from the initial diagnosis of a life-limiting disease, including for patients undergoing adjuvant/neo-adjuvant chemotherapy, or life-threatening illness, during palliative chemotherapy and/or other oncology treatments [10].
Study setting
Study participants were recruited and treated between November 2018 and June 2020 at the Oncology Service of the Clalit Healthcare Services, Lin and Zebulon Medical Centers, Haifa, Israel. The IO Program provides patient-tailored treatments, with the goal of alleviating QoL-related concerns for patients undergoing adjuvant, neo-adjuvant, or palliative oncology treatments. A range of IO treatment modalities are provided by a multidisciplinary team consisting of 23 healthcare practitioners, including integrative physicians and nurses trained in supportive cancer care and integrative medicine, Chinese medicine practitioners, and therapists trained in manual, movement, and mind–body-spirit therapies. All IO practitioners had received extensive training in IO care [11].
Study population and recruitment
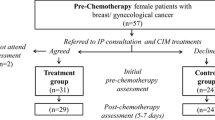
Oncology patients aged ≥ 18 years who were undergoing chemotherapy, biological, endocrine, or radiation therapy for solid tumors were considered eligible for study inclusion. Patients were referred to the IOP team by their oncology healthcare provider (e.g., oncologist, surgeon, oncology nurse, and psycho-oncologist) for QoL-related indications related to their treatment or underlying malignancy. Referred patients were scheduled for an initial 15-min consultation with an integrative physician, with the goal of assessing their QoL-related concerns and the potential value of undergoing IO treatments. Patients were then asked whether they were interested in participating in a study which would compare the short-term (24 h) effects of an IO intervention, provided by IO practitioners alone or with the addition of nurse-guided self-administered treatments (Fig. 1).
Randomization
After providing informed consent, patients were randomly allocated to either the IO intervention or control study arms. Allocation to the study arms was conducted using randomization software (Research Randomizer; randomizer.org) with blocks of 4 and an allocation ratio of 1:1 for the two study arms. Randomization was performed by a research assistant having no contact with study participants, and implemented using sequentially numbered containers. Patient recruitment and enrolment, as well as randomized delegation to either intervention or control groups, were performed by another research assistant. No blinding procedure was used for the present study.
Study interventions
At the end of the initial 15-min integrative physician consultation, treatment goals for both study groups were co-defined with the patient, and an IO treatment plan designed. The primary IO modality implemented was a semi-structured acupuncture protocol, with a set of fixed acupuncture points (colon-4, liver-3, stomach-36, and spleen-6) as well as individualized points, chosen according to the patient’s leading health-related concerns. Acupuncture was combined with breathing/relaxation exercises and manual/movement therapies, in accordance with the patient’s preference. IO treatment sessions lasted approximately 30 min each and were provided by healthcare professionals from the IO program team.
At the end of each treatment session, the IO practitioners provided instruction to patients from both study arms on the self-administration of IO modalities, to be performed at least once during the ensuing 24-h period. Self-administered treatments included at least one of the following modalities: self-acupressure (applying pressure to acupuncture points with the patient’s fingertip); a brief breathing/relaxation exercise; and the preparation of herbal remedies aimed at relieving two of the leading health concerns identified by the patient.
Patients randomized to the intervention arm received additional guidance on the self-administration of IO treatments by the study palliative care nurses who had undergone a 120-h IO training program. The nurse-delivered guidance was provided in person during the IO treatments in addition to the IO practitioner’s instruction, with 24-h follow-up conducted via telephone.
Assessment of study outcomes
The severity of cancer-related fatigue was assessed as the study’s primary outcome. The selection of fatigue as the primary outcome was largely due to the limited availability of effective conventional medicine therapies for this concern [12], as well as previous research supporting the effectiveness of complementary and integrative medicine for this concern [13]. In the present study, patients were recruited if they had attended the initial consultation, regardless of their baseline ESAS fatigue score. This approach reduced the risk of regression to a mean associated with the inclusion of only patients with high fatigue intensity. In addition to fatigue designated as primary outcome, secondary study outcomes included the impact of the intervention on other QoL-related symptoms, such as appetite, pain, sleep, depression, and anxiety, as well as the patient’s self-rated concerns and level of well-being.
Study outcomes were scored in both study groups at baseline; during the initial assessment with the integrative physician; and via telephone at 24 h post-treatment. The decision to assess fatigue and other QoL-related symptoms was based on previous studies examining outcomes as early as 24–48 h following an integrative oncology intervention [14]. In cases where patients could not be contacted at 24 h, a second phone assessment was conducted up to 48 h post-treatment, asking them to score QoL-related outcomes as they recalled at 24 h.
Fatigue was measured using the Edmonton Symptom Assessment Scale (ESAS), which is regarded a leading short-term patient-reported outcome measures in many IO and palliative centers in Israel and the USA, such as the University of Texas M. D. Anderson Cancer Center [15, 16]. The ESAS scores 10 symptoms from the previous 24-h period, using a numerical rating scale ranging from 0 (none) to 10 (worst possible severity). ESAS fatigue is scored from 0 (no fatigue) to 10 (worst fatigue imaginable), with a 1-point decrease in symptom severity considered to be clinically significant [17]. Other symptoms, including appetite, pain, sleep, depression, and anxiety, were also measured using the ESAS.
Patients’ self-rated concerns and well-being were captured by the Measure Yourself Concerns and Wellbeing (MYCAW) questionnaire [18]. The MYCAW tool asks patients to list their two most significant concerns, scoring from 0 (not bothering me at all) to 6 (bothers me greatly). Post-treatment (in the present study at 24 h), MYCAW questionnaires included two additional open-ended questions asking patients to describe the most important aspect of their IO treatment. These were considered short narratives and were qualitatively analyzed using a content analysis approach, precluding the need for pre-established coding categories [19].
Patients’ attitudes toward complementary therapies were assessed with a standardized questionnaire used routinely at the IOP by integrative physicians during the initial consultation. The questionnaire was comprised of 14 items, including questions on whether complementary therapies were being used outside of the IO setting and before being referred to an IO consultation, and patients’ perceptions of the effectiveness and risks of complementary therapies. Patients’ demographics and oncology-related characteristics were also assessed.
Data analysis
The OpenEpi program (Microsoft) was used to determine the sample size required for to identify a significant change in the primary study outcome, fatigue. It was concluded that at least 170 patients were needed, allowing for an alpha-error of 0.05 and beta-error of 0.2 (power = 80%), to identify a 20% delta on the 11-point fatigue scale, when comparing intervention and control groups between baseline and 24-h assessment.
Statistical analyses were conducted with the IBM SPSS Statistics 24.0 program (IBM, New York, NY), with the use of means and standard deviation (SD) or medians and inter-quartile range (IQR) for continuous variables, and numbers and proportions for categorical variables. p-values of < 0.05 were regarded as statistically significant. The chi-square test (for categorical variables) and independent t-test (parametric) or Mann–Whitney (non-parametric) for continuous variables were used to determine demographic differences between study groups and to assess change from baseline to 24-h follow-up on study outcomes.
Ethical considerations
The study protocol was approved by the Ethics Review Board (Helsinki Committee) of the Carmel Medical Center in Haifa, Israel (CMC-18–0139). The study was registered at ClinicalTrials.gov (NCT03676153). Participants signed an informed consent form before entering the study.
Results
Characteristics of study groups
A total of 363 patients were assessed for eligibility, of which 353 were randomized and allocated to either the intervention (n = 187) or control (n = 166) arms of the study (Table 1, Fig. 1). The majority of patients enrolling in the study was females (80.4%), with a predominance of breast, gynecological, and gastrointestinal cancer diagnosis (only 16.2% had another cancer site diagnosis). Both groups had similar baseline demographic and oncology-related characteristics, as well as prevalence of reported complementary therapy use. Participants in the intervention group, however, were more likely to be female (p = 0.031) and perceived complementary therapies to be less risky than controls (p = 0.036). Of the 353 patients randomized at baseline, 286 (81%) completed the 24-h follow-up assessment. Reasons given for not completing follow-up assessment are presented in Fig. 1.
Integrative oncology treatments
In both study groups, acupuncture was the most frequently used IO modality, followed by treatment and instruction (by IO practitioners) on self-administered techniques with manual therapies, herbal medicine, and mind–body therapies (Table 2). Patients in the intervention group received significantly more manual treatments and guidance (63.6% vs. 38%; p < 0.001), with no significant difference regarding other IO modalities. Patients in the intervention group were also more likely than controls to have received two or more IO modalities (68.9% vs. 46.9%; p < 0.001).
Fatigue and other symptoms
A comparison of ESAS scores between the two study groups, from baseline to 24-h follow-up, is presented in Table 3. Between-group analysis showed a significantly greater decrease in fatigue levels at follow-up in the intervention group when compared to controls (p = 0.026). Significantly greater improvement was also found in the intervention arm for appetite (p = 0.003), though this finding was limited by the presence of a significant difference in baseline scores between the two groups (p = 0.048). Patients in the intervention group reported significantly improved pain from baseline to follow-up (p = 0.003, within-group analysis), with no change seen in the control group. Sleep and anxiety scores improved significantly in both study groups, with no between-group difference found.
Twenty-four-hour MYCAW assessment
Patients in both intervention and control groups reported significant baseline-to-24-h within-group improvement for MYCAW symptom scores (p < 0.001), as well as scores for general wellbeing (intervention, p = 0.014; controls, p < 0.001). However, this was non-significant for between-group outcome comparisons.
Qualitative analysis of the MYCAW short narratives highlighted the impact of the additional nurse-guided instruction, emphasizing the ability to implement IO modalities at home more effectively and easily, particularly self-acupressure. Patients described experiencing “confidence, power and strength,” and reported reduced fatigue, abdominal pain, and nausea, as well as improved appetite, breathing, and sleep. At the same time, they also described a “calmness of the soul and spirit…release of blockages” and their satisfaction with “strengthening the body” and “letting the body calm and rest.” Self-treatment was also experienced as a re-visiting of what was perceived as a positive interaction with the IO practitioner and nurse during the in-person treatment session at the cancer center: “I remember doing the exercises she recommended for me…doing meditation, talking to myself, and sitting during sunset in front of the sea… in my corner.”
Many of the patient narratives focused on the intensity of the self-administered treatments implemented at home. While some described a “dose–effect impact on my energy,” others felt the acupressure treatments at the cancer center were more effective than what they were doing at home: “Performing the exercises is hard for me, since I need to think beforehand. If there would be something easier, I would be willing to do it.” Other barriers to self-treatment included “pain interfering with performing the exercises recommended by the nurse,” and a sense of “intense tiredness.”
Safety-related concerns
No major adverse effects were reported in either of the study groups throughout the entire study period. Local and mild adverse effects were noted with acupuncture, including localized discomfort that rarely radiated to more peripheral areas of the treatment. Hyper- or hypoesthesia, primarily numbness and tingling around the acupuncture needle or during acupressure, was reported as well. These effects, however, are both expected and desirable in Chinese medicine and are considered as “de-Qi” sensations, which reflect a therapeutic quality of the treatment.
Discussion
The randomized controlled trial described took place in a setting in which both intervention and control groups underwent an identical IO treatment program, within the framework of an evidence-based, standard-of-care protocol. The study findings indicate that the introduction of IO-trained palliative care nurses to the multidisciplinary IO treatment program, providing additional instruction to patients on self-treatment with IO modalities, is feasible. The guidance provided by these nurses was associated with greater short-term (24 h) scores for fatigue and appetite in patients undergoing active oncology treatment, though further quantitative research is needed. This finding is particularly significant since cancer-related fatigue is extremely prevalent and challenging for oncologists, with currently available therapeutic options limited in their effectiveness, with the exception of physical exercise interventions [20]. This contrasts greatly with other QoL-related symptoms such as pain, nausea, constipation, and diarrhea, for which conventional medicine offers a number of effective, evidence-based treatment options.
The additive impact of the IO-trained nurse-guided instruction needs to be better understood. It is possible that non-specific effects were generated by the additional guidance provided by the IO-trained nurses, which affirmed the guidance provided by the IO practitioners. The nurse intervention may have enhanced patients’ perceptions of care and compassion, especially by involving two (as opposed to one) IO-trained practitioners. The nurse-mediated guidance may have also promoted a sense of empowerment, as suggested in the short MYCAW patient narratives. At the same time, the presence of specific effects should be recognized, and not only those which are non-specific.
It should be noted that both groups of oncology patients received the same IO treatment regimen, provided by IO practitioners, most frequently including acupuncture. The predominance of acupuncture and manual therapies in the present study are similar to what has been found in previous pragmatic IO studies [21]. At the same time, all of the study IO practitioners provided instruction to for the self-administration of at least one IO modality (manual, mind–body, or herbal related) at home during the 24-h period following treatments at the study center. It is possible that the more frequent use of manual/acupressure modalities in the intervention group was related to the addition of nurse-delivered guidance on these practices. Further research is needed to explore why the intervention was shown to be of potential benefit for only ESAS fatigue and appetite scores, and not other QoL-related concerns.
Nurses have the potential to play a leading role in the IO setting: providing the therapies themselves, managing programs, and promoting patient and caregiver education, assessment, and follow-up. In contrast to nurses, IO practitioners are often seen more as healthcare providers when administering treatments in which the patient is more passive (e.g., acupuncture), as opposed to being “teachers” when providing more patient-involved therapies (e.g., yoga). It is possible that the nurse’s role in the intervention group extends beyond the limits of the therapeutic process, toward their role as “teachers” who regularly provide guidance and hands-on instruction to patients at home. Patient education and counseling are central to the nursing profession, and these skills may have helped enhance their ability to serve as “teachers” far more significantly than the IO practitioners. Despite their limited experience in providing IO therapies, they may have enhanced and extended the IO therapeutic effect through the self-treatment process.
Self-administration of IO therapies is increasingly becoming the subject of clinical research, though the effect of these interventions remains to be assessed. Molassiotis et al. conducted a randomized controlled trial that demonstrated the feasibility and safety of acupuncture self-needling as maintenance therapy for cancer-related fatigue, following acupuncturist-delivered treatment [22]. Zick et al. investigated self-administered acupressure for persistent cancer-related fatigue in breast cancer survivors, and found the intervention improved fatigue when compared with usual care, along with sleep quality and QoL [23]. The shift toward a more patient-guided treatment approach, with self-administered therapy, has become even more relevant during the current COVID-19 pandemic [24]. This process has been facilitated by the creation of online practice recommendations, such as those published by the Online Task Force of the Society for Integrative Oncology [5].
Study limitations
The present study has several methodological limitations, most importantly the lack of rigorous control (e.g., use of sham/wait-list controls) and the absence of blinding which may have influenced the study outcomes. The IO treatment and guidance protocol was also not fully structured, incorporating a patient-tailored and multi-modal IO approach. However, this pragmatic approach is far more reflective of the “real-world” IO clinical setting, where treatment protocols are semi-structured and attuned to patients’ QoL profile, expectations, and health beliefs. In addition to the above limitations, the study outcomes were measured only over a short-term (24 h) period, raising the need for future research to assess more long-term effects of the intervention over 1 to 6 weeks following treatment, especially regarding insomnia, anxiety, and other QoL-related concerns. Further research is also needed to address additional patient-reported outcomes, using not only short-term tools such as the ESAS, which examines the past 24 h, but also longer-term tools, such as the EORTC QLQ-C30, which measures QoL over the previous week. The studies will need to examine different IO settings, in other countries and in more diverse populations, to allow for generalizability of the findings. Another study limitation to consider is the potential recruitment bias, which may explain the high enrolment of females in the study as well as the predominance of breast, gynecological, and gastro- intestinal cancer diagnosis. Finally, the effectiveness and safety of the nurse-guided IO therapies need to be addressed, using both quantitative outcome measures and qualitative methodologies to assess the perspectives of nurses, patients, and their caregivers.
In conclusion, the study findings suggest that additional guidance provided by IO-trained nurses (in addition to that of IO practitioners) on the self-administration of IO therapies at home is feasible, as well as providing a short-term additive effect in reducing fatigue and improving appetite. Further research is needed to identify the specific and non-specific effects of nurse-led guidance, as well as the value of creating a continuum of care from the cancer center to the patient’s home, enhancing patients’ accessibility to IO care.ssessments.
Data availability
Data transparency is available pending to request from the submitting author.
Code availability
N/A.
Abbreviations
- QoL:
-
Quality of life
- IO:
-
Integrative oncology
- ESAS:
-
Edmonton Symptom Assessment Scale
- MYCAW:
-
Measure Yourself Concerns and Wellbeing
References
Ben-Arye E, Schiff E, Zollman C, Heusser P, Mountford P, Frenkel M, Bar-Sela G, Lavie O (2013) Integrating complementary medicine in supportive cancer care models across four continents. Med Oncol 30(2):511. https://doi.org/10.1007/s12032-013-0511-1
Witt CM, Balneaves LG, Cardoso MJ, Cohen L, Greenlee H, Johnstone P, Kücük Ö, Mailman J, Mao JJ. A comprehensive definition for integrative oncology. J Natl Cancer Inst Monogr. 2017 2017(52). https://doi.org/10.1093/jncimonographs/lgx012
Lyman GH, Greenlee H, Bohlke K, Bao T, DeMichele AM, Deng GE, Fouladbakhsh JM, Gil B, Hershman DL, Mansfield S, Mussallem DM, Mustian KM, Price E, Rafte S, Cohen L (2018) Integrative therapies during and after breast cancer treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. J Clin Oncol 36(25):2647–2655. https://doi.org/10.1200/JCO.2018.79.2721
Ben-Arye E, Portalupi E, Keshet Y, Bonucci M, Can G, Kading Y, Samuels N, Livas M, Gressel O, Silbermann M, Breitkreuz T (2021) Enhancing palliative care with mindful touch: impact of a manual and movement therapy training program in an international multidisciplinary integrative oncology setting. J Pain Symptom Manage 61(2):229–236. https://doi.org/10.1016/j.jpainsymman.2020.08.004
Ben-Arye E, Paller CJ, Lopez AM, White S, Pendleton E, Kienle GS, Samuels N, Abbawaajii N, Balneaves LG (2021) The Society for Integrative Oncology Practice Recommendations for online consultation and treatment during the COVID-19 pandemic. Support Care Cancer 29(10):6155–6165. https://doi.org/10.1007/s00520-021-06205-w
Spencer CN, Lopez G, Cohen L, Urbauer DL, Hallman DM, Fisch MJ, Parker PA (2016) Nurse and patient characteristics predict communication about complementary and alternative medicine. Cancer 122(10):1552–9. https://doi.org/10.1002/cncr.29819
Ben-Arye E, Schiff E, Raz OG, Samuels N, Lavie O (2014) Integrating a complementary medicine consultation for women undergoing chemotherapy. Int J Gynaecol Obstet 124(1):51–54. https://doi.org/10.1016/j.ijgo.2013.07.019
Klafke N, Mahler C, von Hagens C, Uhlmann L, Bentner M, Schneeweiss A, Mueller A, Szecsenyi J, Joos S (2019) The effects of an integrated supportive care intervention on quality of life outcomes in outpatients with breast and gynecologic cancer undergoing chemotherapy: results from a randomized controlled trial. Cancer Med 8(8):3666–3676. https://doi.org/10.1002/cam4.2196
Ben-Arye E, Shulman B, Eilon Y, Woitiz R, Cherniak V, Shalom Sharabi I, Sher O, Reches H, Katz Y, Arad M, Schiff E, Samuels N, Caspi O, Lev-Ari S, Frenkel M, Agbarya A, Admi H (2017) Attitudes among nurses toward the integration of complementary medicine into supportive cancer care. Oncol Nurs Forum 44(4):428–434. https://doi.org/10.1188/17.ONF.428-434
World Health Organization. (2013). WHO definition of palliative care. Retrieved from http://www.who.int/cancer/palliative/definition/en Accessed: December 20, 2021
Ben-Arye E, Schiff E, Shapira C, Frenkel M, Shalom T, Steiner M (2012) Modeling an integrative oncology program within a community-centered oncology service in Israel. Patient Educ Couns 89(3):423–429
Thong MSY, van Noorden CJF, Steindorf K, Arndt V (2020) Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol 21(2):17. https://doi.org/10.1007/s11864-020-0707-5
David A, Hausner D, Frenkel M (2021) Cancer-related fatigue-is there a role for complementary and integrative medicine? Curr Oncol Rep 23(12):145. https://doi.org/10.1007/s11912-021-01135-6
Ben-Arye E, Hausner D, Samuels N, Gamus D, Lavie O, Tadmor T, Gressel O, Agbarya A, Attias S, David A, Schiff E (2022) Impact of acupuncture and integrative therapies on chemotherapy-induced peripheral neuropathy: a multicentered, randomized controlled trial. Cancer 128(20):3641–3652
Bruera E, Kuehn N, Miller M, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliative Care 7(2):6–9
Oldenmenger, W., de Raaf, P., de Klerk, C., van der Rijt, C. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symp Manage. 2012;pii: S0885–3924(12)00340–5. https://doi.org/10.1016/j.jpainsymman.2012.06.007
Hui D, Shamieh O, Eduardo PC et al (2015) Minimal clinically important differences in the Edmonton Symptom Assessment Scale in Cancer Patients: a prospective, multicenter study. Cancer 121(17):3027–3035
Paterson C, Thomas K, Manasse A, Cooke H, Peace G (2007) Measure Yourself Concerns and Wellbeing (MYCaW): an individualised questionnaire for evaluating outcome in cancer support care that includes complementary therapies. Complement Ther Med 15(1):38–45
Keshet Y, Schiff E, Samuels N, Ben-Arye E (2015) Giving voice to cancer patients: assessing non-specific effects of an integrative oncology therapeutic program via short patient narratives. Psychooncology 24(2):169–174
Chapman EJ, Martino ED, Edwards Z, Black K, Maddocks M, Bennett MI. Practice review: evidence-based and effective management of fatigue in patients with advanced cancer. Palliat Med. 2021 2692163211046754. https://doi.org/10.1177/02692163211046754
Segev Y, Lavie O, Stein N, Saliba W, Samuels N, Shalabna E, Raz OG, Schiff E, Ben-Arye E (2021) Correlation between an integrative oncology treatment program and survival in patients with advanced gynecological cancer. Support Care Cancer 29(7):4055–4064
Molassiotis A, Bardy J, Finnegan-John J, Mackereth P, Ryder WD, Filshie J, Ream E, Eaton D, Richardson A (2013) A randomized, controlled trial of acupuncture self-needling as maintenance therapy for cancer-related fatigue after therapist-delivered acupuncture. Ann Oncol 24(6):1645–1652. https://doi.org/10.1093/annonc/mdt034
Zick SM, Sen A, Wyatt GK, Murphy SL, Arnedt JT, Harris RE (2016) Investigation of 2 types of self-administered acupressure for persistent cancer-related fatigue in breast cancer survivors: a randomized clinical trial. JAMA Oncol 2(11):1470–1476. https://doi.org/10.1001/jamaoncol.2016.1867
Ben-Arye E, Keshet Y, Gressel O, Tapiro Y, Lavie O, Samuels N (2021) Being in touch: narrative assessment of patients receiving online integrative oncology treatments during COVID-19. Support Care Cancer 29(8):4819–4825. https://doi.org/10.1007/s00520-021-06026-x
Acknowledgements
We thank Ms. Lili Perlman and Dr. Dorit Weis, the chief nurses at the Clalit Health Services Central Office, as well as Ms. Rut Baruch and Ms. Ahuva Tal, Chief nurses of the Haifa and Western Galilee district and Carmel Medical Center, Israel, for their vision and support of the 120-h IO nurse training for palliative care nurses. We thank all nurses undergoing the IO training, the Integrative Oncology Program practitioners, and the oncology teams of oncologists, surgeons, nurse oncologists, and psycho-oncologists in Lin, Zebulun, and Carmel medical centers. We thank Ms. Ronit Leiba for the statistical analysis.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Eran Ben-Arye, Orit Gressel, Bella Shulman, Yehudit Tapiro, and Ilanit Shalom Sharabi. Analyses were performed by Eran Ben-Arye, Orit Gressel, and Noah Samuels. The first draft of the manuscript was written by Eran Ben-Arye, Lynda Balneaves, and Noah Samuels, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocol of the study was approved by the Ethics Review Board (Helsinki Committee) of the Carmel Medical Center in Haifa, Israel; and was registered at ClinicalTrials.gov (NCT01860365).
Consent to participate
Participation in this study was voluntary and verified by participants’ consent.
Consent for publication
All authors consented for publication of the present manuscript.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben-Arye, E., Balneaves, L.G., Yaguda, S. et al. Nurse-guided patient self-treatment in integrative oncology: a randomized controlled trial. Support Care Cancer 31, 233 (2023). https://doi.org/10.1007/s00520-023-07689-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07689-4