Abstract
Objectives
Cancer is a leading cause of death. This paper examines the utilisation of unscheduled emergency end-of-life healthcare and estimates expenditure in this domain. We explore care patterns and quantify the likely benefits from service reconfigurations which may influence rates of hospital admission and deaths.
Methods
Using prevalence-based retrospective data from the Northern Ireland General Registrar’s Office linked by cancer diagnosis to Patient Administration episode data for unscheduled emergency care (1st January 2014 to 31st December 2015), we estimate unscheduled-emergency-care costs in the last year of life. We model potential resources released by reductions in length-of-stay for cancer patients. Linear regression examined patient characteristics affecting length of stay.
Results
A total of 3134 cancer patients used 60,746 days of unscheduled emergency care (average 19.5 days). Of these, 48.9% had ≥1 admission during their last 28 days of life. Total estimated cost was £28,684,261, averaging £9200 per person. Lung cancer patients had the highest proportion of admissions (23.2%, mean length of stay = 17.9 days, mean cost=£7224). The highest service use and total cost was in those diagnosed at stage IV (38.4%), who required 22,099 days of care, costing £9,629,014. Palliative care support, identified in 25.5% of patients, contributed £1,322,328. A 3-day reduction in the mean length of stay with a 10% reduction in admissions, could reduce costs by £7.37 million. Regression analyses explained 41% of length-of-stay variability.
Conclusions
The cost burden from unscheduled care use in the last year of life of cancer patients is significant. Opportunities to prioritise service reconfiguration for high-costing users emphasized lung and colorectal cancers as offering the greatest potential to influence outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a leading cause of death worldwide [1]. Whilst ‘death and dying are inevitable’ [2], the moments leading to death create the causes and conditions for the development of meaning, thereby constructing a peaceful or traumatic transition. Palliative care underpins symptom management in the end of life and is considered a human right [3], since its absence can lead to unnecessary suffering [4]. Given the rise in cancer incidence in coming decades [5], and the fact that end-of-life expenditure and utilisation of healthcare services are peak [6, 7] (up to 20% of NHS budgets [8]), it is essential to examine how patients can receive care throughout their cancer pathway, into palliation.
Improving outcomes during end of life is grounded in patients’ use of and experiences in healthcare services. Realising preferences for home-based [9] and individualised end-of-life care are recommended [10]. Discussions of preferred place of death support the management of care complexity [11, 12]. However, difficulties finding information on advanced planning care [13], potentially exacerbate dissonance between stated and revealed preferences and lead to a significant proportion of patients dying in hospital [14, 15].
Those approaching end of life should expect coordinated care [16]; however, service gaps can lead to emergency hospital admissions which are not always necessary or medically indicated [17]. Such admissions alongside other quality indicators (short interval between chemotherapy and death, high proportion of hospital deaths vs home deaths, frequent emergency room visits, high number of hospital/Intensive Care Unit days near the end of life and hospital admission near to death) can identify healthcare systems delivering aggressive or poor-quality care [18]. Furthermore, readmissions believed undesirable are associated with care quality during initial hospital stays, transitional care and post-discharge support [19]. Consequently, 30-day readmission rates and admissions in the last 28 days of life may be meaningful metrics in examining coordination issues. Conversely, indicators of good-quality care, communication, shared decision-making, advance directives and pain and symptom management [18], are infrequently available within patient datasets to examine end-of-life experiences.
Palliative care reduces symptom burden, improves satisfaction and quality of life of patients and caregivers and potentially extends the length of life [20]. Economic studies addressing palliative care suggest savings result from fewer hospitalisations and reduced use of acute care. Notably, hospital palliative care input has been associated with a reduction in readmission [21].
Palliative care access and ‘in-home’ procedures may prevent emergency admissions given the symbiotic relationship between primary and tertiary care [17]. The appropriate method for controlling symptoms in some patients will be hospital-based; however, the difference between hospital visits indicating necessary care versus aggressive care varies [22]. Therefore, to target and scale palliative care service development and identify those likely to benefit from service improvement, critical data insights are needed.
Thus, we evaluate the cost characteristics of a Macmillan-funded report [15] commissioned to examine unscheduled care use before the introduction of acute oncology services in 2016. This study examines the length of stay (LOS) costs and assesses for potential predictors of LOS in cancer patients in Northern Ireland (NI). Study results support the prioritisation of services, delivered in the right place, to the right patient, which may reduce avoidable emergency admissions and hospital-based deaths.
Methods
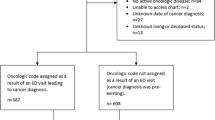
We conducted a retrospective cost consequence study using a prevalence-based approach by secondary data analysis, including all patients who died in 2015 in NI of cancer (ICD10 C00-C97) and report in line with guidelines [23]. Within the secure environment of the Northern Ireland Cancer Registry, anonymised data extracted from the NI General Registrar’s Office was linked (by cancer diagnosis) to Patient Administration System episodes for unscheduled emergency care (UEC) admissions between 1st January 2014 and 31st December 2015; these data were collected to capture all UEC episodes experienced in the last year of life for each cancer patient to examine patterns of how unscheduled care services were being used before the introduction of acute oncology teams.
In the initial report of this data [15], there were higher rates of emergency admissions for haematological, brain and CNS, lung and mesothelioma cancers. Infection was cited as the most common cause of admissions (23.9%). Of those admitted for emergency care in the last year of life, 10.8% had been diagnosed with cancer on the same day. Of the emergency admissions, 33.4% took place during normal working hours. The report provided a limited examination of LOS, identifying that 1 in 5 (20.5%) had a total inpatient stay of more than 28 days.
The primary aim of our study was to calculate a cost scenario by the LOS outcomes, from the payer perspective [24]. Since these anonymised data provided diagnostic codes but limited detail of specific care performed during admission, micro-costing was not possible. Additional data mining provided codes to cost palliative care support and GP/ambulance involvement for each admission.
Clinical expert input verified that a direct palliative care admission was not standard practice during the data extract period; therefore, in line with costing recommendations, costs were included per bed day [25]. All calculations are based on ‘in-year’ (2015) estimated national average costs, shown in Table 1. Analyses were conducted in ‘R’.
We conducted (multiple) linear regressions (n=15 models detailed in Appendix Table 5) to examine variation in the length of stay. We examine heterogeneity and identify if there were significant predictor variables of LOS (using the ‘lm’ function in R). We examined patient-level attributes, disease stage, type, age and gender, and independently evaluated service-level attributes to examine differences in LOS by palliative care exposure. To assess quality outcomes from a patient perspective, we examined admissions in the last 28 days of life, 30-day readmissions and place of death. In patients with two tumours recorded (n=19), the total number of care episodes and LOS only were included in analyses.
Finally, hypothesising that expanded use of alternative care models results in reduced use of acute care, we modelled the cost savings by assuming that time patients spend in acute care/hospital could be decreased by either a reduction in the number of UEC admissions (by 5, 10, 15, 20, 25%) or a reduction in the mean LOS in hospital following an admission (by 1, 3 or 5 days) compared to the baseline, as previously deployed in other exemplary reports [26].
Results
In 2015, 4224 patients died with a recorded cancer diagnosis. Of the patients, 97.9% had complete data for analysis. Of these, 3134 patients (74.1%) were admitted for a total of 60,746 days (averaging 19.5 days) of inpatient UEC during their last year of life; 48.9% (n= 1535) had one or more UEC admissions during their last 28 days of life. A total of 6058 episodes of care were delivered. Most cases were admitted to general medicine units (47.6% of first admissions), with the remainder admitted to surgical (12.3%), oncology (5.5%), haematology (2.8%), general assessment (2.3%), urology (1.4%), fractures (1.2%), gastroenterology (1.0%), ENT (0.9%) and cardiology (0.9%) units. We found small numbers of ICU admissions n=<10. Intensive rescue activities were found in <1% of cases within the dataset, details shown in Appendix Table 6. The total LOS for ICU-identified cases was 19 days. There were 1008 episodes where patients had readmissions within 30 days (Appendix—“30-day readmissions”).
The total estimated cost to the health service for unscheduled care was £28,684,261, averaging £9200 per person admitted. Inpatient specialist palliative care (support) was identified in 800 records (25.5% of those admitted). Where palliative care input was indicated in an UEC record, the additional cost, as attributed per bed day, contributed £1,322,328 (or 4.6%) of total costs, averaging £1653 per person identified. Ambulance transfers (n=89) contributed £21,004 to the total estimated costs, with a further £18,971 in identifiable GP referral costs for those (7.9%) of patients identified as having a preceding GP referral recorded. Additional LOS costs by age, gender, socio-economic status and admissions in the last 28 days of life and place of death are given in Appendix—Fig. 2, “Gender”, “Socio-economic group”, Table 7 and Fig. 3.
Age
Of those admitted, 0–19-year-olds (n<10) had the greatest mean inpatient days (25.3 days, standard deviation (SD) 32.4 days) with mean average costs of £9030, followed by 80–89-year-olds (n=649; mean =20.1 days, mean cost = £7855). Third highest mean LOS was in 60–69-year-olds (n=782, 19.9 days, mean cost = £7822), followed by 70–79-year-olds (n=938, 19.5 days, mean cost = £7718), and 90+-year-olds (n=131, mean = 18.9 days, mean cost = £7399) and 40–49-year-olds (n=148, 18.7 days, mean cost = £7530). Those aged 20–29 and 50–59 years old (n=17 and n= 399 respectively) had the lowest mean days (both 17.9 days, mean cost = £7118 and £7189 respectively) with slightly higher mean LOS in 30–39-year-olds (n=46, 18.1 days, mean cost = £7213). In line with absolute costs, the largest overall total inpatient days were amongst those aged 70–79 years (see Appendix—Fig. 2).
Stage of disease
The highest service use and total cost were in those diagnosed with stage IV disease (n= 1198; 38.4%), requiring 22,099 days of inpatient UEC, with an attributed LOS cost of £9,629,014 (Fig. 1). Notably, the mean LOS was 18.4 days (mean cost = £7377, SD= £5821), in this group, which was the lowest. Those with an unknown stage at diagnosis had the second greatest utilisation of UEC (n=960) which required 19,851 days of UEC, costing £8,649,511 with a mean inpatient stay of 20.7 days. Those who presented with stage III disease (n=542) used 10,311 days of UEC, averaging 19.0 days and costing £4,492,726. Those with stage II (n=259) used 4927 days of UEC, costing £2,146,801. The lowest overall service use and total costs for UEC in the last year of life were amongst those with stage I disease at diagnosis (n=159, used 3421 days UEC). However, the mean LOS was greatest in those with stage I disease, with 21.5 days, with the highest mean cost (£8293, SD= £6866) at an overall cost of £1,490,604.
Tumour type
Lung cancer patients had the highest proportion of admissions for UEC (23.2%, n=788), with a mean LOS of 17.9 days (SD=17, mean cost = £7224, SD= £5418, see Table 2) requiring 14,078 total days of UEC, at a total cost of £6,134,090. The second-highest use was in colorectal and anal cancer patients (11.4%, n= 364), requiring 6901 days of UEC at a total cost of £3,006,915, with a mean LOS of 19 days (SD=17, mean cost = £7565, SD= £5700), followed by oesophageal and stomach cancer patients (7.1%, n=238) 4295 days of UEC at a total cost of £1,871,425 (mean LOS = 18 days, mean cost pp = £7300, SD= £5079). Full details by cancer type are shown in Table 2. The mean inpatient days (for UEC) were greatest in small intestine cancer patients (30.3 days), and lowest in non-melanoma skin cancer patients (NMSC, 10.9 days), with parallel highest mean cost in small intestine cancers (£10,807) and lowest mean cost in those with NMSC (£4846).
Palliative care as a factor influencing length of stay
Specialist palliative care support was recorded as provided to 800 patients during admissions in their last year of life, requiring 11,112 days of UEC provision, costing £1,322,328. The mean cost of care for those who did not receive palliative care was £6959, and £9849 for those who did. We compared three observed variations of palliative care support received as a potential indicator of efficacy, in those (a) with further admissions noted afterwards, (b) who died before discharge and (c) with no subsequent admissions.
Linear regression of differences in LOS using those with no palliative care as the reference value showed relative differences between coefficients in the groups (Table 3). Relative to the intercept (18.4), group A (with further admissions) had 9.7 additional days of UEC, group B (those who died before discharge) had 3.2 additional days and those in group C (with no further admissions) had −0.8 days relative to those with no palliative care recorded during admissions.
Attributes affecting length of stay
Iterative addition of patient-level characteristics and service variables (age, stage, gender, palliative care input, place of death) were examined by regression to estimate the variability in LOS (detailed in Appendix, Table 5). In primary analyses, which included all cases including those not admitted for care, up to 41% of the variability in LOS was accounted for (a maximum of 14 variables included). When restricted to only those who received UEC, only 15% of the variability of the LOS could be accounted for.
Potential impact of reduction in emergency admissions or length of stay
From the baseline of £28,684,281 total costs and a mean LOS of 19.5 days, a reduction of 1 day in mean LOS without any additional reduction in the overall number of admissions would release £1,520,267 (Table 4). On average, a 3-day reduction of mean LOS, combined with a 10% reduction in admissions, implies £7.37 million reductions in the UEC cost in the last year of life. The maximum reduction hypothesised, with a 5-day reduction of mean LOS accompanied by a 25% reduction in admissions, would potentially release £14.71 million in UEC costs.
Discussion
To the best of our knowledge, our results provide the first estimates of the cost consequences of unscheduled emergency care admissions in cancer patients in the last year of life in Northern Ireland, showing that ~three in four patients required admission during the last year of life, averaging 19.5 days of care. More than 60,000 days of unscheduled emergency care were delivered. Concerningly almost half of those patients experienced unscheduled inpatient care during the last 28 days of life.
Despite a substantive cost burden of ~ £28 million, less than 5% of costs were contributed by specialist inpatient palliative care input. Data showed a minority of patients (25.5% of those admitted) accessed palliative care, which is significantly lower than other reports, where up to 64.5% of cancer patients had at least one palliative care event [27]. Although individual patients may have displaced activity in other sectors, the level of access or availability in our cohort remains concerning, considering the high proportion of deaths in hospitals and the high numbers accessing care in the last 28 days of life. These results highlight the need for priority action since evidence suggests specialist palliative and end-of-life care can reduce hospital admissions [22, 28], and could reduce the likelihood of costly and aggressive treatments in the last days of life, where patients can access palliative care at an early stage [27].
More than one in three of those admitted was diagnosed with stage four disease, which required £9.6 million in UEC costs. In keeping with global trends [29], the largest proportion of patients (one in four) admitted had lung cancer, and are understood to be at risk of later diagnosis (67.8% likely to be diagnosed at an advanced stage [30]). Whilst these patients had a lower mean LOS (17.9 days) than the overall average, their total attributable cost was greatest (~£6 million). Thus, prompt consideration of the return on investments in lung health checks [31] should be prioritised. Importantly, recent supportive trial data on low-dose CT screening for early diagnosis of lung cancer indicate mortality benefits [32]. Attendant ‘time to benefit’ estimates remain several years; thus, prompt consideration of building appropriate capacity is vital, due to costs associated with the status quo of late diagnosis of potentially avoidable cancers.
Concerningly, patients with colorectal cancers were the second-highest users of UEC, costing more than £3 million; efforts to redistribute stage and refine outcomes for this group should be priority policy decisions. In light of the significant morbidity and mortality in this disease [33], where there are existing screening programmes for early diagnosis, expanded access to the screening process with more effective disease detection testing thresholds might improve patient outcomes [34] and prompt reductions in UEC.
Although those 0–19 years had the highest average days and costs of care, those aged 70–79 years had the greatest total costs of care (£7.9 million). Therefore, targeting the LOS in children and young adults may not leverage the same benefit as intervening in those aged 70–79 years. Our result concurs with a recent valuation of informal care to cancer decedents at end of life which showed that the mean age of recipients was 70–79 years [35]. This study identified the monetary value of informal care at £948.86 per week, derived from the relative costs at the wage rates of those who might otherwise complete the care (the proxy good method). They report that care was in the majority (65%) delivered by females, 55% of whom were previously in employment. The implications identify the significant shortfall in the replacement cost of their care relative to Carers’ Allowance payments (not including lost income and collateral costs on productivity in this valuation). The attendant implications for our study highlight the potential for displacement effects shown by our results, where informal carers were unable to meet their needs. Many cannot afford or indeed may be supported by employers to adopt a carer role in the order of the hours reported (93 h per week) [35]. Thus, some of those 1535 people in our study who sought care in the last 28 days of life may reflect choices that stem from practical considerations and difficulties in hospice care access. The next appropriate step for this cohort would be to match data on primary care and scheduled care use, staffing ratios and detail on formal or informal carer access, to examine if age-based use of services is mediated by these factors rather than the disease characteristics, co-morbidity [36] or palliative and hospice care provisions.
Finally, the paucity of data on inpatient care constrained the characterisation of (quality) markers of aggressive care or palliative care at the end of life provided, as drivers of LOS. Although regression models explained 41% of LOS variation, evidently, factors not included in the data greatly influence LOS. Whilst staffing ratios may be a factor [37], their attributable weighting is unclear. Moreover, many administrative datasets do not provide standardised reporting; therefore, minimum data sets and indicator development should seek to harmonize across jurisdictions [38], since a review of palliative care costing in the UK [39] suggested lacking data has implications on how palliative care (and palliative care research) is planned and funded. Thus, developing accurate coding for palliative care services, accurately mapped to patient-centred outcomes and factors attributed to higher levels of congruence in preferred place of care [40], are essential to ensure critical insights and evaluations are possible to enable international benchmarking and to redress inequity and improve patient experiences at the end of life.
Limitations
Recognising service displacement, future work should consolidate healthcare use trajectories reflecting the sequence of patient episode data (primary and planned care) to complement the insights described. However, a review of palliative care costs showed that hospital costs accounted for the greatest proportion of the overall cost of care [39]. We are therefore reassured to have prioritised the area most likely to reflect the greatest overall costs, and thereby, trends herein are likely to convey greater relevance in clinical optimisation decision-making.
Within the administrative admissions dataset, it was impossible to distinguish between acceptable interventions and potentially unnecessary ‘rescue’ care, but there were low levels of intensive activities overall. Critically, with the data available, it was not possible to identify how many days within an episode palliative care was contacted; therefore, it was not possible to fully characterise (specialist palliative) care input. Necessarily, our approach will result in a lower estimated level of gross expenditure than the real-world scenario but may result in an overestimate of absolute palliative care days provided. Thus, relative differences in LOS cannot be used to determine palliative care efficacy and are exploratory only.
Conclusions
Unscheduled care use in the last year of life of cancer patients poses a significant time burden for patients costing acute health services ~ £28 million. Existing data infrastructure limits the ability to fully characterise causality and quality aspects of this care. Opportunities to focus service reconfiguration and early detection efforts for those with the highest cost use, prioritised in lung and colorectal cancers, would provide the greatest potential to reduce care burdens.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
UK National Palliative and End of Life Care Partnership 2015 Ambitions for palliative and end of life care: a national framework for local action 2015-2020. 1–6.
World Health Organisation 2020 Palliative care 1–5.
Cruz-Oliver DM (2017) Palliative care: an update. Mo Med 114:110–115
Donnelly D, Gavin A (2015) Cancer incidence trends 1993-2013 with projections to 2035. https://www.qub.ac.uk/research-centres/nicr/FileStore/PDF/NIrelandReports/Filetoupload,532183,en.pdf
Yabroff KR, Lund J, Kepka D, et al. 2014 Economic burden of cancer in the US. Cancer Epidemiol Biomarkers Prev DOI: 10.11: 1–18
Diernberger K, Luta X, Bowden J et al (2021) Healthcare use and costs in the last year of life: a national population data linkage study. BMJ Support Palliat Care 1–8. https://doi.org/10.1136/bmjspcare-2020-002708
Georghiou T, Bardsley M (2014) Exploring the cost of care at the end of life. https://www.nuffieldtrust.org.uk/files/2017-01/end-of-life-care-web-final.pdf. Accessed 16 Sept 2019
Office for National Statistics (2016) National Survey of Bereaved People (VOICES) 2015 Quality of care delivered in the last 3 months of life for adults who died in England. 1–20
Evans R, Finucane A, Vanhegan L et al (2014) Do place-of-death preferences for patients receiving specialist palliative care change over time? Int J Palliat Nurs 20:579–583
Capel M, Gazi T, Vout L et al (2012) Where do patients known to a community palliative care service die? BMJ Support Palliat Care 2:43–47
Arnold E, Finucane AM, Oxenham D (2015) Preferred place of death for patients referred to a specialist palliative care service. BMJ Support Palliat Care 5:294–296
Marie Curie (2021) Public attitudes to death and dying in the UK, https://www.mariecurie.org.uk/globalassets/media/documents/policy/public-attitudes-to-death-and-dying-report-final.pdf. Accessed 11 Nov 2021
Marie Curie Cancer Care (2013) Death and dying: understanding the data. 1–36
Cairnduff V, Dwyer L, Burns C, Fallica G, Shiell- Davis K, Fox CAG (2019) Macmillan-NICR partnership: emergency admissions in last year of life for people dying of Cancer in Northern Ireland in 2015, https://www.qub.ac.uk/research-centres/nicr/FileStore/PDF/Filetoupload,914678,en.pdf. Accessed 16 Sept 2019
National Institute for Health Care Excellence (2021) End of life care for adults: service delivery. Quality Standard 13:1–17
Hjermstad MJ, Kolflaath J, Løkken AO et al (2013) Are emergency admissions in palliative cancer care always necessary? Results from a descriptive study. BMJ Open 3:e002515
Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZBS (2003) Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 21:1133–1138
Friebel R (2018) What do changes in readmission rates tell us about quality of care in the NHS?, https://www.health.org.uk/blogs/what-do-changes-in-readmission-rates-tell-us-about-quality-of-care-in-the-nhs. Accessed 26 Aug 2019
May P, Cassel JB (2018) Economic outcomes in palliative and end-of-life care: current state of affairs. Ann Palliat Med 7:S244–S248
May P, Garrido MM, Del Fabbro E et al (2019) Evaluating hospital readmissions for persons with serious and complex illness: a competing risks approach. Med Care Res Rev 77(6):574–583
Henson LA, Gao W, Higginson IJ et al (2015) Emergency department attendance by patients with cancer in their last month of life: a systematic review and meta-analysis. J Clin Oncol 33:370–376
Husereau D, Drummond M, Augustovski F et al (2022) Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ 376. https://doi.org/10.1136/BMJ-2021-067975
Department of Health 2016 Reference Costs 2015-16. Dep Heal 1–59
Department of Health (2016) Reference costs guidance 2015-16, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/497127/Reference_costs_guidance_2015-16.pdf. Accessed 21 June 2019
Hatziandreu E, Archontakis F, Daly A (2008) The potential cost savings of greater use of home- and hospice- based end of life care in England, https://www.rand.org/content/dam/rand/pubs/technical_reports/2008/RAND_TR642.pdf. Accessed 24 July 2019
Ziegler LE, Craigs CL, West RM et al (2018) Is palliative care support associated with better quality end-of-life care indicators for patients with advanced cancer? A retrospective cohort study. BMJ Open 8:1–9
Seow H, Brazil K, Sussman J et al (2014) Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 348:1–10
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Barclay ME, Abel GA, Greenberg DC et al (2021) Socio-demographic variation in stage at diagnosis of breast, bladder, colon, endometrial, lung, melanoma, prostate, rectal, renal and ovarian cancer in England and its population impact. Br J Cancer 124:1320–1329
Cancer Research UK 2021 Lung Health Checks. 1–6.
Field JK, Vulkan D, Davies MPA et al (2021) Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg Heal - Eur 10. https://doi.org/10.1016/j.lanepe.2021.100179
Henderson R, French D, Maughan TS et al (2021) Defining the economic burden of colorectal cancer across Europe: a population-based cost-of-illness study. Lancet Gastroenterol Hepatol 6:709–722
McFerran E, O’Mahony JF, Naber S et al (2022) Colorectal cancer screening within colonoscopy capacity constraints: can FIT-based programmes save more lives by trading-off more sensitive test cut-offs against longer screening intervals? MDM Policy Pract 7:1–12
Urwin S, Van den Berg B, Lau YS et al (2021) The monetary valuation of informal care to cancer decedents at end-of-life: evidence from a national census survey. Palliat Med 35:750–758
Diernberger AK, Luta X, Bowden J, et al. Variation in hospital cost trajectories at the end of life by age, multimorbidity and cancer type. Epub ahead of print 2022. DOI: https://doi.org/10.1101/2022.02.22.22271323.
Lasater KB, Aiken LH, Sloane D et al (2021) Patient outcomes and cost savings associated with hospital safe nurse staffing legislation: an observational study. BMJ Open 11:1–8
Langton JM, Blanch B, Drew AK et al (2014) Retrospective studies of end-of-life resource utilization and costs in cancer care using health administrative data: a systematic review. Palliat Med 28:1167–1196
Gardiner C, Ryan T, Gott M (2018) What is the cost of palliative care in the UK? A systematic review. BMJ Support Palliat Care 8:250–257
García-Sanjuán S, Fernández-Alcántara M, Clement-Carbonell V et al (2022) Levels and determinants of place-of-death congruence in palliative patients: a systematic review. Front Psychol 12:1–12
Data availability
The author(s) acknowledge the contribution of the Northern Ireland Cancer Registry staff in the production of the Northern Ireland Cancer Registry data. Like all Cancer Registries, this work uses data provided by patients and collected by the health service as part of their care and support—data requests should be directed to the NICR. Anonymised data were collected from the Northern Ireland Cancer Registry, managed by restricted access to EMF/VC on secure encrypted devices; full details of security and acceptable use are available here. No patient-identifiable information was shared.
Funding
This research was conducted using data from the Northern Ireland Cancer Registry (NICR) which is funded by the Public Health Agency of NI (VC, AG). The primary author (EMF) was funded by grants from Cancer Focus Northern Ireland and Health Data Research UK. VC was funded through the Macmillan-NI Cancer Registry Partnership (2016). ML received grant funding from his roles as Associate Director of Health Data Research Wales-Northern Ireland and Scientific Director for DATA-CAN, The Health Data Research Hub for Cancer. The interpretation and conclusions of the data are the sole responsibility of the authors.
Author information
Authors and Affiliations
Contributions
1. Made a substantial contribution to the concept or design of the work, or acquisition, analysis or interpretation of data—EMF, VC
2. Drafted the article or revised it critically for important intellectual content—EMF, VC, RE, AG, ML
3. Approved the version to be published—EMF, VC, RE, AG, ML
4. Have participated sufficiently in the work to take public responsibility for appropriate portions of the content—EMF, VC, RE, AG, ML
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study used secondary data sources, the study received approval from the Northern Ireland Cancer Registry, and no patient-level consent was required.
Consent for publication
In line with the above approvals, Northern Ireland Cancer Registry staff verified study outputs to ensure there were no risks of accidental identification of patients by the data characteristics presented for publication, in line with confidential disclosure practices.
Competing interests
ML declares honoraria from Bayer, Carnall Farrar, EMD Serono, Novartis, Pfizer and Roche unrelated to this work. The other authors declare that there are no conflicts of interest.
Additional information
Key statements
What is already known about the topic?
• People with palliative care needs are high users of unscheduled care in the last months of life.
• Cost savings from early palliative care accrue due to both reduced length of stay and reduced intensity of treatment.
What this paper adds
• In their last year of life, 3 out of 4 cancer patients used unscheduled emergency care (averaging 19.5 days of care) costing ~£28million, averaging £9200 per person admitted.
• 48.9% of those who received unscheduled care had ≥1 admission during their last 28 days of life.
Implications for practice, theory or policy
• Patients with lung and colorectal cancers were the leading users of unscheduled care, thus the opportunity to advance policies for earlier diagnosis with improved survival could drive forward reductions in service demands.
• Short-term measures to reduce the length of stay, such as ‘acute oncology’ teams intended to focus on inpatient users of care, could potentiate significant cost savings, offering the potential for reallocation of spending to early diagnosis services.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
30-day readmissions
In total, there were 1008 episodes where readmission within 30 days of a previous admission occurred (1.65% of all admissions), with 226 individuals having multiple episodes of 30-day readmissions during their last year of life. Although not directly applied in the final cost model, we calculated a hypothetical readmission ‘penalty’ or tariff incurred, as a marginal difference between one additional day of excess bed day costs on the LOS of the previous admission and the cost of a new admission Day 1 cost, which was equal to £1303 per episode. Thus, for these (1008) events, where patients were readmitted on days 2-30 after a prior admission, the consequent hypothetical additional UEC cost ‘penalty’ of these 30-day readmissions was £1,313,424. This amount reflects a minimum cost since it does not reflect the duration of the readmission LOS.
Gender
Of those accessing UEC, the minority were female (n=1394) compared with (n=1740) male. Whilst a higher proportion of males (75.7%) had at least one emergency admission recorded in the last year of life compared to females (72.5%; p=0.018), the mean LOS was similar - for females 19.2 days compared with 19.6 days for males (accounting for 44.1% and 55.3% of total inpatient days respectively). The total cost for UEC in the last year of life was £11,638,126 for females and £14,605,391 for males. The total LOS for females (26,710 days) was lower than for males (33,520 days). Mean costs for females £7,632 (SD = £5,490) was similar to males (£7,720, SD= £6313).
Socio-economic group
Although there were no statistically significant differences by socio-economic groups, there were greater numbers of people admitted for UEC in the last year of life from the most deprived socio-economic group (SES5 n=680, 21.6%, total cost £5,691,397, Table 7) as compared to the least deprived (SES1) n=586 (total cost £4,896,205). In SES2 (n=643) total UEC cost was £5,395,542), SES3 (n=605) total UEC cost was £5,335,848, SES4 (n=599) total UEC cost was £5,069,622. However, the mean UEC cost was highest in SES3 (£7,975) while the lowest average UEC cost was in SES2 (£7,516).
Admissions in the last 28 days of Life & Place of Death
Admissions in the last 28 days of life and place of death. Of those (n=3134) people admitted during the last year of life, we found n=1535 patients had admissions in the last 28 days of life. For this, we total the final admission length of stay for each person. The final admissions, where the admission is noted to have occurred within the last 28 days of life, require a total of 18,638 days of unscheduled care. Based on this, we estimate the cost of unscheduled care delivered to those within the last 28 days of life was £7,694,212 (~ 26.8% of total costs, mean cost pp = £5012). Of those who had an admission within the last 28 days of life, n=371 (24.2%) had received palliative care within one or more of their unscheduled care episodes, and n=530 people (34.5%) died before discharge. Comparing within those (n=530) people who died before discharge, 71.5% did not have palliative care involvement documented in their unscheduled care record (n=379 people). An analysis of the data by the place of death shows that those who went on to die in hospice care (n=455) had the greatest average time from diagnosis to death (927 days) and the lowest average UEC cost in the last year of life £6680 per person; total cost in this group was £3,039,381 (Fig 3). Those who went on to die in nursing home care (n=270) had an average time from diagnosis to death of 814 days and had the highest average cost of UEC in the last year of life £9555 per person, total cost £2,579,831 (note here we do not explicitly know if the persons were ordinary residents in nursing homes pre-/during and post-diagnosis). Those who went on to die at home (n=966) had the shortest average time from diagnosis to death of 739 days, average cost £6876 and total cost £6,642,295. In those who died in hospital (n=1494, 47% of those with UEC episodes), the average time from diagnosis to death was 752 days, with total costs of £12,733,465, with an average cost of £8523 in their final year of life.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McFerran, E., Cairnduff, V., Elder, R. et al. Cost consequences of unscheduled emergency admissions in cancer patients in the last year of life. Support Care Cancer 31, 201 (2023). https://doi.org/10.1007/s00520-023-07633-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07633-6