Abstract
Purpose
To evaluate the acceptability, satisfaction, and preliminary efficacy of cognitive training for improving cognitive function and health outcomes in breast cancer survivors (BCS).
Patients and methods
BCS enrolled in this 2-group randomized, double-masked controlled trial of cognitive training. Primary outcomes included the acceptability and satisfaction of the interventions. Secondary outcomes included examining the effect size and reliable improvement of perceived cognitive function and health outcomes, including work ability, health perception (status and change), and quality of life. Exploratory outcomes were performance on neuropsychological tests and plasma levels of brain-derived neurotropic factor (BDNF). Data were collected at baseline and immediately post-intervention. Using ANCOVA models, the intervention was compared to attention control while adjusting for covariates and baseline values. The effect sizes for differences in means and the reliable improvement percentage were reported.
Results
Thirty-six BCS completed the study and were on average 57.6 (SD = 8.0) years old, 59.4% Caucasian, and had some college education (74.5%). Both programs were reported to be satisfactory and acceptable. Non-significant small effect sizes were noted for the intervention on cognitive abilities (d = 0.26) and cognitive concerns (d = − 0.32), with reliable improvement noted in 32% and 28% of BCS, respectively. Small to medium effect sizes were noted in improvement in work ability (d = 0.37) and health perception status (d = 0.30) and change (d = 0.60, p < 0.05).
Conclusions
Cognitive training was acceptable to BCS and resulted in improvement in perceived cognitive function and perceptions of “real-world” health benefits. A larger randomized controlled trial is warranted to determine its effectiveness for objective cognitive performance.
Similar content being viewed by others
Introduction
Cancer-related cognitive impairment (CRCI) in breast cancer survivors (BCS) has been well-documented as a late and long-term effect of cancer and its treatment [1]. In fact, up to 75% of BCS report cognitive concerns [2,3,4] which have also been documented on neuropsychological exam [5]. Most importantly, CRCI has been shown to have ramifications for everyday life. Researchers have shown that CRCI is disruptive for BCS in their ability to work [6, 7], perceptions of health, and overall quality of life [2, 8]. This has made identifying a satisfactory and efficacious treatment option imperative.
Cognitive training interventions have shown some promise in recent studies and may be a viable treatment option [9, 10]. In a recent review, Fernandes and colleagues (2019) noted that cognitive rehabilitation approaches for CRCI, including cognitive training, resulted in improvements in at least one cognitive measure (self-report or performance-based testing) [9]. Although these findings suggest the importance of cognitive training, these trials have been limited by designs using usual care [11] or wait-list control comparators, which fail to address placebo effects [12, 13]. Thus, these trials have failed to assess the acceptability, satisfaction, and preliminary efficacy of cognitive training compared to active attention control on cognitive outcomes. In addition, these trials have failed to fully examine transfer effects on “real-world” or “everyday” implications such as impact on self-rated work ability, health perceptions, and quality of life. Previous studies in the well elderly however have noted improvements in self-rated health [14] and quality of life [15]. For cognitive training to be fully evaluated in BCS, research is needed to not only examine direct effects on cognitive function but also assess transfer effects of training on everyday functioning (work ability) and health perception [9].
Therefore, the purpose of this pilot study was to evaluate the acceptability, satisfaction, and preliminary efficacy (effect sizes) of cognitive training versus active attention control for improving perceived cognitive function and “real-world” health outcomes in BCS. Primary outcomes included the acceptability and satisfaction with delivering the intervention remotely for both the intervention (computerized cognitive training) and active attention control (computerized games). Secondary outcomes included determining what if any effect cognitive training has on perceived cognitive function and health outcomes, including work ability, health perception (status and change), and quality of life. Finally, exploratory outcomes included objective cognitive performance on neuropsychological tests of episodic memory, attention and working memory, speed of processing, and verbal fluency/executive function as well as levels of plasma brain-derived neurotropic factor (BDNF), which is related to neuroplasticity and cognitive function [16]. Overall, findings from this study are intended to inform a full-scale efficacy trial and advance our overarching goal of identifying an effective treatment for cognitive impairment in BCS.
Patients and methods
Study design
This double-masked, randomized controlled trial compared cognitive training (BrainHQ) to attention control among BCS. Outcomes were assessed at baseline (prior to randomization) and post-intervention. The study protocol was approved by the Institutional Review Board. Although the trial was stopped early due to the interruption caused by the COVID-19 pandemic, the study achieved its primary goal to examine the acceptability and satisfaction of the cognitive training for CRCI in BCS.
Procedure and methods
BCS were recruited from a Midwestern NCI-designated Cancer Center and affiliated clinics, direct mailings via Cancer Center registry, and advertising through survivorship newsletters or local events. Eligible participants were BCS who reported concerns regarding their cognitive functioning (poor memory, feelings of mental slowness, etc.) and were ≥ 21 years of age, ≥ 6 months post-treatment (except for anti-estrogen treatment) which included chemotherapy for primary non-metastatic breast cancer (stage I–IIIA), disease-free, and able to understand, speak, read, and write English. BCS were excluded if they had a previous diagnosis that would impact neuropsychological function (e.g., history of stroke, traumatic brain injury, brain surgery, dementia, Alzheimer’s disease, or Parkinson’s disease; history of cranial radiation therapy or intrathecal therapy).
BCS who consented completed a baseline neuropsychological assessment, survey questionnaires, and blood draw (T1). Subjects were then stratified by age (≤ 50 and ˃ 50) and randomly assigned to one of two groups: cognitive training (BrainHQ) or attention control (computerized games including puzzles and word searches) and were asked to complete a total of 40 h of training over 10 weeks. The post-intervention assessment occurred immediately after completion of the 10-week intervention (T2). All assessments were conducted in-person in the same manner with repeat neuropsychological testing, questionnaires, and blood collected by a trained and blinded staff member. Participants received $20 and parking at each data collection visit (total $40) to offset participant burden.
Cognitive training intervention versus attention control
Cognitive training included the utilization of the commercially available BrainHQ program (Posit Science) [17]. This program systematically reduces the stimulus duration during a series of progressively more difficult information-processing tasks presented via computer. The exercises automatically adjust to user performance to maintain an 85% correct rate. The exercises included time-order judgment, discrimination, spatial-match, forward-span, instruction-following, and narrative-memory tasks [18].
The attention control group was assigned to complete online activities via the computer as well. The attention control program is a computer-based general cognitive stimulation intervention (computerized crossword puzzles) that offers a pre-determined set of computerized crossword puzzles and word puzzles delivered from the same platform and format as the intervention and has been successfully used in other NIH-funded cognitive training trials [15]. The delivery format, number of sessions, and setting were the same as the intervention group.
Outcome measures
All of the outcome measures used in this study are reliable, valid, and have been used in BCS population, including our previous studies [12]. Cronbach’s alpha for all survey scales in this study was 0.77 and above.
Primary outcomes
Acceptability and satisfaction were assessed post-intervention with the 8-item, Likert-based Client Satisfaction Questionnaire [12, 19]. BCS were asked to rate items (e.g., overall satisfaction, quality of training, etc.) on a 4-point scale (total score range 8–32), with higher scores indicating greater acceptability and satisfaction.
Secondary outcomes
Perceived cognitive functioning was measured with the PROMIS Cognitive Abilities and Cognitive Concerns 8-item, 5-point Likert scales [20]. The Cognitive Abilities Scale items target positive self-assessments of cognitive functioning with higher scores indicating better cognitive ability. The Cognitive Concerns Scale items are worded negatively and express concerns in the same area with higher scores indicating more cognitive concerns.
Health outcomes included work ability, perceptions of and changes in health, and quality of life (general mental health). Work ability: Work ability was assessed by one item from the Work Ability Index (WAI) [21]. This item assessed current work ability on a Likert scale from 1 (cannot work at all) to 10 (work ability at its best) with higher scores indicating better work ability. General Health Perception and Quality of life (general mental health): The Medical Outcomes Study-Short-Form Health Survey (SF-36) general perceptions of health status subscale was used to measure general health perceptions (5 items), changes in general health perception (1-item), and general mental health (5-items), on a 5-point Likert scale, with higher scores indicating positive perceptions of general health and general mental health [22].
Exploratory outcomes
Objective cognitive performance and BDNF
Neuropsychological tests included measures of learning and episodic memory (Rey Auditory Verbal Learning Test [Rey AVLT] [23] and Rivermead Behavioral Paragraph Recall Test [24]), attention and working memory (Digit Span, total raw score from the WAIS-III [25]), speed of processing (Symbol Digit Modalities Test [SDMT], Oral Response Version [26]), and verbal fluency/executive function (Controlled Oral Word Association [COWA] Test) [27]. Plasma levels of BDNF were measured in duplicate by ELISA (catalog number DBD00; R&D Systems, Minneapolis, MN). The minimum detectable level of BDNF in this assay is 20 pg/ml, and the within-assay variation is 4.2% at 1339 pg/ml.
Statistical analysis
Group equivalence on baseline characteristics was tested using chi-square tests, Fisher’s exact tests, and Wilcoxon rank sum tests. Satisfaction and acceptability scores were summarized by group. The Mantel–Haenszel test of linear trend was used to compare groups. Neuropsychological tests were standardized by pooling scores across time points and groups for all subjects using the Blom (rank-based) transformation, producing more normally distributed scores [28]. Standard z scores were computed at each time point. Separate ANCOVA models were used to test cognitive training effects compared to attention control on each outcome. Models included between-subject treatment along with age, education, tamoxifen use, and the baseline value for the outcome variable. The treatment effect size was computed as the difference between model-based adjusted means at post-intervention divided by the pooled baseline standard deviation. Ninety-five percent confidence intervals of effect size were obtained by fitting the model to two thousand bootstrap samples obtained by sampling with replacement. The 2.5th and 97.5th percentiles of the distribution of effect size were used as confidence interval limits. Ranges for Cohen’s d of small effect size as 0.2, medium effect size as 0.5, and large effect size as 0.8 were used [29]. Reliable improvement was calculated as improvement in performance on a measure by at least 1 standard error of measurement (SEM). The SEM described generally in Dudek [30] was computed as the standard deviation of difference scores (from baseline to post-intervention for the attention control group multiplied by the square root of 1 minus test–retest (baseline to immediate post-intervention) reliability) for the attention control group. There was no missing neuropsychological data and less than 0.02% of questionnaire data. Analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC). The significance level was not adjusted for multiple comparisons because this was a pilot study.
Results
Participants
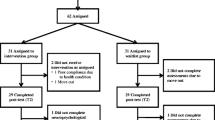
A total of 36 BCS completed the entire study and were included in the analyses. Figure 1 shows the CONSORT diagram including information on accrual flow and reasons for attrition. A total of 50 BCS provided consent to participate; however, 4 BCS (8%) withdrew before the T1 baseline assessment. Reasons for withdrawing prior to baseline included time constraints with scheduling the baseline assessment (3 BCS) and travel for data collection (1 BCS). A total of 46 BCS were randomized to cognitive training (22 BCS) and attention control (24 BCS). Study completion rates by group were 86% (n = 19) cognitive training and 75% (n = 18) attention control. Completion rates for in-person T2 assessments were disrupted due to COVID-19. Although 18 BCS completed the attention control, 1 BCS was eliminated from the data analysis due to a reported and documented significant neurological diagnosis between T1 and T2, leaving a total of 17 subjects in the analyses.
The overall sample (collapsed across treatment groups) consisted of middle-aged (average 57.6 + / − 8.0 years old) White (59%) and Black (41%) women who had some college education (74.5%). In addition, most had early-stage breast cancer (78% stage II or lower) and were long-term survivors (average of 6.1 years post-treatment (SD = 4.9)). Most BCS had surgery (89%) and chemotherapy (100%) and 83% also had radiation therapy. Almost half the subjects (44%) were receiving anti-estrogen therapy at the time of this study.
There were no significant group differences at baseline in age, race, cancer treatment (including the use of aromatase inhibitors), depressive symptoms, anxiety, fatigue, sleep disturbance, perceived cognitive abilities and cognitive concerns, or BDNF levels. Education level and tamoxifen use were trending toward being significantly different between the two groups. We controlled for age and level of education, known covariates of cognitive performance, and tamoxifen use because endocrine therapy has been shown to impact cognitive outcomes in previous research [31]. Most of the cognitive tests were also similar except for speed of processing (SDMT), with the intervention group performing significantly worse than the attention control group (p = 0.026). Immediate memory (p = 0.073) and delayed memory (p = 0.052) on the Rey AVLT were also noted as being marginally different, with the intervention group worse than attention control; however, these values were accounted for in the statistical analyses by controlling for baseline scores in each model (see Table 1).
Primary outcome—satisfaction and acceptability
There was no significant difference in the retention rate between the two interventions. The number lost to follow-up during the study was most likely impacted by the emergence of COVID-19 and closing of the study; however, importantly, there were no significant differences in satisfaction and acceptability between the computerized cognitive training and active attention control groups (p = 0.37) for those completing the study. Overall ratings were positive for both programs with mean total scores 29.7 (SD = 6.0) and 27.9 (SD = 5.7), respectively. Figure 2 displays the mean values for each individual satisfaction question. The overall satisfaction item showed that 84% BCS in the computerized cognitive training group rated it as satisfactory to highly satisfactory (scores of 3 and 4) and 95% identified that they were likely to highly likely to use the program again if offered (booster sessions).
Secondary outcomes: perceived cognitive abilities and concerns and health outcomes
Results of the primary outcome measures of cognitive abilities, cognitive concerns, and health outcomes, including work ability and health perceptions (status and change), are detailed in Table 2. None of the differences between computerized cognitive training and active attention control were significantly different except for health perception change (p = 0.044). Compared to the active attention control, the computerized cognitive training group demonstrated small effects for improvement in perceived cognitive abilities (d = 0.26) and cognitive concerns (d = − 0.32) immediately post-intervention, with 32% and 28% of the intervention group demonstrating reliable improvement, respectively (Table 2). Small to medium effect sizes were noted in improvement in work ability (d = 0.37) and health perception (status [d = 0.30] and perceived change in health [d = 0.60, p = 0.026]). Notably, 56% of BCS had reliable improvement in work ability in the computerized cognitive training group compared to 27% in the active attention control group. Perception of health, including both health status and perceived change in health, was higher in the computerized cognitive training group compared to active attention control, with 32% versus 24% and 47% versus 12% of BCS reporting improvement, respectively. Computerized cognitive training did not have an effect on quality of life measured by the SF-36 general mental health scale; however, 32% reported reliable improvement post-intervention in the computerized cognitive training group compared to 12% in the active attention control group.
Exploratory outcomes: cognitive function BDNF levels
Table 3 displays the effects of computerized cognitive training on secondary outcomes. No significant differences were noted on the neuropsychological tests between groups. Learning and memory (Rey AVLT), attention and working memory, speed of processing, and verbal fluency/executive functioning all demonstrated a trend for improvement in the intervention group versus control, with effect sizes ranging from d = 0.10 to d = 0.20. The largest reliable improvement was noted on learning and memory (Rey AVLT) with 74% from the intervention group demonstrating improvement versus 53% in the attention control group. BDNF levels did not significantly correlate with cognitive performance at baseline or post-intervention. No appreciable differences were noted in BDNF levels between the two groups.
Discussion
This was the first double-masked RCT to date that remotely tested computerized cognitive training against an active attention control intervention in long-term BCS. This is important as cognitive training intervention studies have been heavily criticized for failing to provide an appropriate active attention control group to fully distinguish satisfaction of training as well as intervention effects of training versus placebo effects [32]. In addition, to bring about meaningful and clinically significant improvement, cognitive training should not only improve the perception of cognitive function, but it should also have transfer effects on important health outcomes, such as work ability, health perception, and quality of life [15].
The main finding of this study was that both the computerized cognitive training and the computerized active attention control (puzzles, crosswords, etc.) programs delivered remotely were well-received. This program appealed to a diverse sample of BCS recruiting a larger minority population (41% Black) and represents a more diverse dissemination than previous in-person cohorts [12], suggesting that remote training is feasible to deliver and more acceptable to a larger population of BCS [33]. In addition, the majority of BCS in both groups reported the training as acceptable and overall satisfying. Identifying acceptable evidence-based interventions for CRCI to support BCS in need is imperative. Lange et al. identified in a web-based survey that most cancer survivors would like to receive supportive treatment (75%, n = 909) and specifically identified cognitive training (72%, n = 658) as a desired option [34]. However, the difficulty faced by clinicians to date is that there has been very little evidence to support the use of cognitive training for CRCI. Thus, these preliminary findings that suggest that the cognitive training is acceptable and satisfying are crucial to the development of larger scale trials to address CRCI.
Importantly, the active attention control served as an acceptable alternative and comparator for interventional research of cognitive training. This is consistent with previous literature that found mind-stimulating interventions, such as puzzles, are engaging [35] and have been identified by BCS themselves as useful in addressing CRCI [36]. Thus, remote delivery and application of computerized cognitive training against computerized attention control seem appropriate for a large full-scale randomized controlled trial.
Although not statistically significant, small and positive effect sizes for cognitive training were noted in both perceived cognitive abilities and cognitive concerns. Similarly, previous studies have demonstrated that cognitive training has a significant and positive effect on improving subjective cognitive improvement in BCS [11, 12]. In addition, this trial noted that intervention effects transferred to improvements in work ability and health perception (health status and health perception change). These transfer effects to “real-world” outcomes are essential. Multiple studies have documented the significant untoward effects of CRCI in BCS [10]. BCS with cognitive concerns often incur difficulty in returning work and performing at work (work ability) [6, 7]. Many BCS refer to work as returning to a sense of normalcy [37] and most must return to work due to financial need and to maintain health benefits [38], making work ability an important outcome target for future interventional studies. In addition, decline in cognitive function has been linked to decrements in both health perception and quality of life [15], whereas cognitive training has been shown to improve quality of life in BCS [12] and healthy older adults [15]. In an earlier study, we noted improvements in BCS in symptom distress (mood disturbance, anxiety, and fatigue) and quality of life using this cognitive training intervention in a lab-based setting [16]. It is hypothesized that cognitive training may have a neurobiological effect, as it operates through sensory-motor elaboration and repetition. The procedural tasks required with this training program may lead to increasing brain activation. On a neural level, such increases in activity reflect a strengthening of the response within a particular region [39]. The effect of enhanced task-related activity, or an increase in the number of neurons being activated during a particular task, is hypothesized to create new networks that are more accurate and less susceptible to interference [40] and ultimately improve cognitive performance and contribute to a sense of well-being noted by the participant [20]. These transfer effects on health and well-being are of great importance for BCS, of which the majority are middle-aged and juggling both work and family obligations. Findings from this study also inform our future intervention research in assessing work ability as sensitive outcome of cognitive training.
In this preliminary study, we also successfully assessed objective cognitive function using measures that represent the cognitive domains that have been shown to be most affected in BCS with CRCI [41]. Overall, there were no significant effects in favor of cognitive training on objective cognitive performance. This finding is in contrast to other preliminary studies in BCS [12, 13], healthy older adults [42], and adults with mild cognitive impairment [43]. In a meta-analysis of 52 studies with a total of 4885 healthy older adult participants, Lampit and colleagues found significant and positive effects in favor of computerized cognitive training over control with specific effect sizes in the small to moderate range for memory, working memory, speed of processing, and visuospatial skills [42]. However, they noted studies using an unsupervised/unsupported home-based administration were less effective [42]. Lampit et al. recommended the need for ongoing technology support and problem-solving of information technology issues for in-home use and methods to enhance training adherence (reminder and motivational cues). Taken together, future research in this area may want to consider more instructional support and also provide reminder cues and technology support during the intervention training to support cognitive training adherence.
BDNF did not correlate with subjective and objective measures of cognitive function at either assessment and did not differ significantly between the two groups. BDNF has been shown to be associated with self-reported cognitive concerns in BCS; however, levels also remained relatively stable over time among individuals with specific polymorphism (Met homozygous carriers of the BDNF rs6265 polymorphism) [44]. Thus, future research exploring this biological marker should not only include plasma levels of BDNF, but also investigate the differences in plasma BDNF levels between the rs6265 genotypes to fully evaluate BDNF as a sensitive biomarker of intervention effects.
Strengths and limitations of the study
There were a number of strengths of this study. Important methodological strengths were the masking of participants and cognitive testers to intervention assignment and comparing cognitive training to an active attention control comparator, and thus addressing placebo effects. The study was limited as it was stopped earlier than planned due to COVID-19; however, it was believed that to continue would have created significant variation in assessments and outcomes (e.g., COVID-19 impact on cognitive concerns, work ability, quality of life/mental health) and the sample size was considered adequate for a pilot study [45].
Conclusion
Cognitive training was shown to be acceptable and satisfactory intervention for CRCI in BCS. Findings suggest some positive effects of training with benefits in perceived cognitive function, work ability, and health perception. These pilot study findings also point to need for a full-scale efficacy trial of cognitive training in addressing CRCI in a larger, diverse sample of BCS.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Ganz PA, Van Dyk K (2020) Cognitive impairment in patients with breast cancer: understanding the impact of chemotherapy and endocrine therapy. J Clin Oncol JCO2000336. https://doi.org/10.1200/JCO.20.00336
Shilling V, Jenkins V (2007) Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs 11(1):6–15. https://doi.org/10.1016/j.ejon.2006.02.005
Janelsins MC, Keler SR, Ahles TA, Morrow GR (2014) Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 26(1):102–113. https://doi.org/10.3109/09540261.2013.864260
Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG et al (2017) Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 35(5):506–514. https://doi.org/10.1200/JCO.2016.68.5826
Janelsins MC, Heckler CE, Peppone LJ, Ahles TA, Mohile KM, Mustian KM et al (2018) Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol 36(32):3231–3239. https://doi.org/10.1200/JCO.2018.78.6624
Boykoff N, Moieni M, Subramanian SK (2009) Confronting chemobrain: an indepth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv 3(4):1932–2267
Von Ah D, Storey S, Crouch A (2018) Relationship between self-reported cognitive function and work-related outcomes in breast cancer survivors. J Cancer Surviv 12(2):246–255. https://doi.org/10.1007/s11764-017-0664-6
Mehnert A, Scherwath A, Schirmer L, Schleimer B, Petersen C, Schulz-Kindermann F et al (2007) The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns 66(1):108–118. https://doi.org/10.1016/j.pec.2006.11.005
Fernandes HA, Richard NM, Edelstein K (2019) Cognitive rehabilitation for cancer-related cognitive dysfunction: a systematic review. Support Care Cancer 27(9):3253–3279. https://doi.org/10.1007/s00520-019-04866-2
Mayo SJ, Lustberg M, Dhillion HM, Nakamura ZM, Allen DH, Von Ah D et al (2021) Cancer-related cognitive impairment in patients with non-central nervous system malignancies: an overview for oncology providers from the MASCC Neurological Complications Study Group. Support Care Cancer 6:2821–2840. https://doi.org/10.1007/s00520-020-05860-9
Bray VJ, Dhillon HM, Bell ML, Kabourakis M, Fiero FM, Yip D et al (2017) Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol 35(2):217–225. https://doi.org/10.1200/JCO.2016.67.8201
Von Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, Yu M et al (2012) Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 135(3):799–809. https://doi.org/10.1007/s10549-012-2210-6
Meneses K, Benz R, Bail JR, Vo JB, Triebel K, Fazeli P et al (2018) Speed of processing training in middle-aged and older breast cancer survivors (SOAR): results of a randomized controlled pilot. Breast Cancer Res Treat 168(1):259–267. https://doi.org/10.1007/s10549-017-4564-2
Wolinsky FD, Vander Weg WM, Martin R, Unverzagt FW, Willis SL, Marsiske M et al (2010) Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci 65(5):591–598. https://doi.org/10.1093/geronb/gbp117
Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Wright E, Tennstedt SL (2006) The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci 61(5):S281–S287. https://doi.org/10.1093/geronb/61.5.s281
Zimmer P, Mierau A, Bloch W, Struder HK, Hulsdunker T, Schenk A et al (2015) Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk Lymphoma 56(2):347–352. https://doi.org/10.3109/10428194.2014.915546
Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL et al (2001) ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials 22(4):453–479. https://doi.org/10.1016/S0197-2456(01)00139-8
Mahncke HW, Bronstone A, Merzenich MM (2006) Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res 157:81–109. https://doi.org/10.1016/S0079-6123(06)57006-2
Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD (1979) Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 2(3):197–207. https://doi.org/10.1016/0149-7189(79)90094-6
Lai JS, Wagner LI, Jacobsen PB, Cella D (2014) Self-reported cognitive concerns and abilities: two sides of one coin? Psychooncology 23(10):1133–1141. https://doi.org/10.1002/pon.3522
Tuomi K, Ilmarinen J, Martikainen R, Aalto L, Klockars M (1997) Aging, work, life-style and work ability among Finnish municipal workers in 1981–1992. Scand J Work Environ Health 23(Suppl 1):58–65
McHorney CA, Ware JE Jr, Raczek AE (1993) The MOS 36-Item Short-Form Health Survey (SF-36) Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31(3):247–63. https://doi.org/10.1097/00005650-199303000-00006
Rey A (1941) L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie 28:286–340
Wilson B, Cockburn J, Baddeley A (1985) The Rivermead Behavioral Memory Test. Reading, England, and Gaylord, Mich: Thames Valley Test Co and National Rehabilitation Services
Wechsler D (1997) WAIS-III administration and scoring manual. The Psychological Corporation, San Antonio
Smith A (1982) Symbol Digit Modalities Test. Los Angeles: Western psychological services
Benton AL, Hamsher KD (1989) Multilingual aphasia examination. AJA Associates, Iowa City
Blom G (1958) Statistical estimates and transformed beta variables. John Wiley & Sons, New York
Cohen J (1988) Statistical analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale, NJ
Dudek FJ (1979) The continuing misinterpretation of the standard error of measurement. Psychol Bull 86:335–337
Wagner LI, Gray RJ, Sparano JA, Whelan TJ, Garcia SF, Yanez B et al (2020) Patient-reported cognitive impairment among women with early breast cancer randomly assigned to endocrine therapy alone versus chemoendocrine therapy: results from TAILORx. J Clin Oncol 38(17):1875–1886. https://doi.org/10.1200/JCO.19.01866
Motter JN, Devanand DP, Doriswamy PM, Sneed JR (2016) Clinical trials to gain FDA approval for computerized cognitive training: what is the ideal control condition? Front Aging Neurosci 8:249. https://doi.org/10.3389/fnagi.2016.00249
Crouch A, Von Ah D, Storey S (2017) Addressing cognitive impairment after breast cancer: what do women want? Clin Nurse Spec 31(2):82–88. https://doi.org/10.1097/NUR.0000000000000279
Lange M, Licaj I, Clarisse B, Humbert X, Grellard JM, Tron L et al (2019) Cognitive complaints in cancer survivors and expectations for support: results from a web-based survey. Cancer Med 8(5):2654–2663. https://doi.org/10.1002/cam4.2069
Fissler P, Kuster OC, Laptinskaya D, Loy S, von Arnim CAS, Kolassa IT (2018) Jigsaw puzzling taps multiple cognitive abilities and is a potential protective factor for cognitive aging. Front Aging Neurosci 10:299. https://doi.org/10.3389/fnagi.2018.00299
Von Ah D, Storey S, Jansen CE, Allen DH (2013) Coping strategies and interventions for cognitive changes in patients with cancer. Semin Oncol Nurs 29(4):288–299. https://doi.org/10.1016/j.soncn.2013.08.009
Peteet JR (2000) Cancer and the meaning of work. Gen Hosp Psychiatry 22(3):200–205. https://doi.org/10.1016/s0163-8343(00)00076-1
Mehnert A, de Boer A, Feuerstein M (2013) Employment challenges for cancer survivors. Cancer Suppl 11:2151–2159. https://doi.org/10.1002/cncr.28067
Poldrack RA (2000) Imaging brain plasticity: conceptual and methodological issues–a theoretical review. Neuroimage 12(1):1–13. https://doi.org/10.1006/nimg.2000.0596
Lustig C, Shah P, Seidler R, Reuter-Lorenz PA (2009) Aging, training, and the brain: a review and future directions. Neuropsychol Rev 19(4):504–522. https://doi.org/10.1007/s11065-009-9119-9
Ahles TA, Root JC, Ryan EL (2012) Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 30(30):3675–3686. https://doi.org/10.1200/JCO.2012.43.0116
Lampit A, Hallock H, Valenzuela M (2014) Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med 11(11):e1001756. https://doi.org/10.1371/journal.pmed.1001756
Hill NT, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A (2017) (2017) Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry 174(4):329–340. https://doi.org/10.1176/appi.ajp.2016.16030360
Ng T, Lee YY, Chae JW, Yeo AHL, Shwe M, Gan YX et al (2017) Evaluation of plasma brain-derived neurotrophic factor levels and self-perceived cognitive impairment post-chemotherapy: a longitudinal study. BMC Cancer 17(1):867. https://doi.org/10.1186/s12885-017-3861-9
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 4(4):287–291
Acknowledgements
The authors would like to acknowledge Danielle Estrada and Nicki Coleman, RN, for their work on the study.
Funding
Center for Enhancing Quality of Life, Indiana University School of Nursing (PI: Von Ah) and the Precision Health Grand Challenge Initiative at Indiana University School of Medicine (PI: Shakar). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Indiana University School of Nursing which funded this trial. The funding agency had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Susan Perkins had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Posit Science Corporation is the developer of the cognitive training program (BrainHQ®) used in this study. Posit Science Corporation holds the patent for and a proprietary interest in this software and provided access to the program for the conduction of the trial.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Drs. Diane Von Ah and Adele Crouch. Data analysis was completed by Susan Ofner and Dr. Susan Perkins. The first draft of the manuscript was written by Dr. Diane Von Ah and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The questionnaires and methodology for this study were approved by the Institutional Review Board at Indiana University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants and included ability to publish data obtained in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Von Ah, D., McDonald, B.C., Crouch, A.D. et al. Randomized double-masked controlled trial of cognitive training in breast cancer survivors: a preliminary study. Support Care Cancer 30, 7457–7467 (2022). https://doi.org/10.1007/s00520-022-07182-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07182-4