Abstract
Purpose
As the cancer survivors increase, patients using long-term and high-dose opioids are also increasing. Therefore, the promotion of appropriate use is important. This study investigated the actual status of opioid prescriptions in Japan and identified factors associated with long-term, high-dose prescription.
Methods
We conducted a case-control study using a hospital-based administrative claims database. Patients with a diagnosis of cancer and prescriptions of opioids were included. Patients who received continuous opioid for less than 183 days were defined as the “control,” and patients who received continuous opioid at higher dose levels (≥ 120 mg/day of oral morphine equivalent) for 183 days or more were defined as the “case.” The case was subdivided into two groups: those with the duration of less than 730 days (case I) and 730 days or more (case II). After describing factors possibly associated with long-term, high-dose opioid prescription, ordinal logistic regression analysis was conducted.
Results
We included 19,176 patients; of these, 13,517 were in the control, 111 were in the case I, and 682 were in the case II. The analysis showed that distant metastasis, back pain, dose of opioids, non-opioid analgesics, prescription, and chemotherapy during the opioid prescriptions were significantly associated with long-term, high-dose opioid prescription.
Conclusion
Four percent of the study population were prescribed long-term, high-dose opioids, and several comorbidities and concomitant medications were identified as associated factors. Opioids might be also prescribed for non-cancer chronic pain. It is necessary to properly distinguish the type of pain and to use opioids safely and appropriately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, the population of cancer patients is growing rapidly with the aging of the population, and the survival time of patients is becoming longer due to progress in early diagnosis and treatment [1, 2].
Cancer pain is associated with decreased quality of life and poor adherence to cancer treatment; therefore, appropriate pain control with opioids is important. When cancer patients survive for a long time, their cancer pain are often complicated by non-cancer pain, and long-term opioid use requires pain-specific treatment while also considering treatment of breakthrough pain [3].
On the other hand, long-term or high-dose prescriptions are more likely to cause the adverse effects such as opioid-induced hyperalgesia, gonadal dysfunction, intestinal dysfunction, cognitive dysfunction, sleep disorders, immune disorders, and addiction. Appropriate use of opioids is also important to prevent the occurrence of an opioid crisis [3].
The study by Jones et al. reviewed the literatures investigating the factors associated with long-term opioid therapy for cancer survivors, and describes a relationship between long-term opioid use and important biopsychosocial factors such as cancer sites, socioeconomic factors, and comorbidities [4]. Whereas although Azuma et al. reported a nationwide survey of opioid use in Japan [5], there are few reports on the actual status of opioid prescriptions among cancer patients in Japan, and the factors that lead to long-term, high-dose prescriptions are not clear. Azuma et al. reported that the adequacy of opioid analgesic consumption was lower in Japan than in the USA or European countries; however, the survival time of cancer survivors is increasing and there is concern that long-term, high-dose prescriptions of opioids might become a problem in Japan. Therefore, it is essential to identify the factors associated with long-term, high-dose opioid use and to lead to appropriate use.
In the present study, we investigated the situation of cancer patients in Japan prescribed opioids and the factors associated with long-term, high-dose prescriptions. We also investigated exploratory associations between rescue medications, which are often used for breakthrough pain, and long-term, high-dose prescriptions of opioids.
Methods
Study design and data source
This study was designed as a case-control study using a hospital-based administrative claims database in Japan constructed by Medical Data Vision Co., Ltd (MDV) [6]. The MDV claims database contains over 30 million patient demographics (age, sex), ICD-10 coded diagnoses, medical practices, hospitalizations, prescribed medications, and drug prices from over 400 hospitals with an acute inpatient care system called Diagnostic Procedure Combination (DPC) covering 22% of Japanese hospitals with a DPC system.
Study population
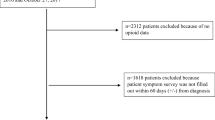
From the MDV claims database, we identified the eligible patients by the following three criteria: (i) having diagnosis of cancer between April 2008 and July 2020 (ICD-10 codes shown in the Supplement), (ii) having at least one prescription of any of the following opioids: morphine, fentanyl, oxycodone, tapentadol, hydromorphine, methadone, and/or buprenorphine on or after the date of the first diagnosis of cancer; and (iii) could be followed for at least 730 days. The definition of opioids was based on the Clinical Guidelines for Cancer Pain Management [7]. The injectable preparations were excluded from the definition of opioid because they are not expected to be utilized for the long-term pain control, and buprenorphine transdermal patches (Norspan Tape®) are not indicated for cancer pain in Japan. To enroll patients for whom the duration of opioid prescription could be assessed, the eligibility criteria for the follow-up period were considered. The follow-up period was defined as the duration between the index date which was the date of the first prescription of opioids after the initial diagnosis of cancer and the date when the patient died or the last record on his/her care was available.
Analysis population
The analysis population was defined based on the duration of opioid prescription and the mean opioid dose during the prescription period. The definitions of the cut-off values for long term and high dose were based on previous studies. The definition of long-term opioid prescription was defined by referring to the clinical guideline for non-cancer pain [8]. The guideline states that “after 6 months of opioid treatment with a good response, a dose reduction or drug holiday should be discussed with the patient.” Since the target patients of this study were cancer patients but the pain was not limited to cancer pain, patients who were prescribed the drug for more than 6 months (183 days) were defined as long term. The definition of high-dose opioid use follows that of “Guidance on the appropriate use of medical narcotics” published by Ministry of Health, Labour, and Welfare of Japan and “Guidelines for Pharmacologic Management of Neuropathic Pain” by Japan Society of Pain Clinicians [3, 9], which define ≥120 mg per day of oral morphine equivalence as a high dose.
Patients for whom opioids were prescribed continuously for less than 183 days were allocated to the control group, and patients having received prescription of opioids for 183 days or more at the mean dose level ≥120 mg per day of oral morphine equivalence were allocated to the case group. To assess the impact of further long-term prescriptions, the case group was subdivided into two groups according to the opioid prescription period: those with the prescription period of less than 730 days (case I) and 730 days or more (case II).
The prescription period was defined as the duration from the index date to the date of death or end of the prescription. The end of prescription was defined as an interval of 30 days or more between prescriptions of the relevant opioid after the index date, and the last day covered by the last prescription was defined as the day of end of opioid prescription. Even when the opioid was changed to other opioid(s) or combined with additional opioid(s), the prescription was considered to be continued (Fig. 1).
The mean opioid dose during the prescription period was calculated by dividing the active ingredient’s quantity equivalent in potency to the oral-dose morphine hydrochloride hydrate 30 mg (an opioid) prescribed during the prescription period [3, 7] by the prescription period.
Statistical analysis
Factors associated with long-term, high-dose prescription of opioid
The following factors possibly associated with long-term, high dose prescription of opioids were described separately for the case groups and the control group: gender, age, site of cancer, metastasis, comorbidities, dose of opioids, duration from diagnosis of cancer to the start of opioid prescription, prescription of pain-related drugs other than opioids, and types of cancer treatment. Prescription of pain-related medication other than opioids and types of cancer treatment were evaluated both before and after the start of opioid prescription. Each case group was compared with the control group in a multiple comparison using the Dunnett test for continuous variables and the Steel test for categorical variables.
On the basis of the descriptive statistics, ordinal logistic regression analysis was conducted to investigate the factors associated with long-term, high-dose prescription of opioids. The control group, case I group, and case II group were considered as dependent variables. Through this analysis, the odds ratio of long-term, high-dose prescription of opioids for each explanatory variable and its 95% confidence interval were calculated. The explanatory variables were selected by the following steps. Step 1: selection of variables of descriptive statistics showing significant differences both between the control group and case I group and between the control group and case II group (p<0.05) as well as categorical variables whose incidence in the case or control group was 5% or higher. Step 2: variables selected from step 1 were re-evaluated for co-linearity and clinical validity.
Assessment of the rescue medication prescription
To investigate the actual status of rescue medication, the present study additionally evaluated the rescue medication during the opioid prescription in each of the case and control groups. The rescue medication prescription was defined as prescription of oxycodone (Oxinorm®) powder, oral immediate release tablet, hydromorphone (Narurapid®) tablets, fentanyl (Abstral®) sublingual tablets, fentanyl (E-fen®) buccal tablets, morphine (Anpec®) suppositories, morphine (Opso®) oral solution, or morphine hydrochloride immediate release tablet. The percentage of patients who received rescue medication during the prescription period of opioid was calculated. In addition, among patients who received rescue medication, the mean daily number of rescue medication use, which is the total number of rescue medication use during the opioid prescription period divided by the prescription period, and the mean rescue medication dose, which is the total doses of rescue medication during the opioid prescription period divided by the opioid prescription period, were described. Each case group was compared with the control group in a multiple comparison using the Steel test for percentage of patients receiving rescue medication and the Dunnett test for the mean daily number of rescue medication use and the mean rescue medication dose.
For all calculations of oral morphine equivalent dose, fentanyl sublingual tablets and fentanyl buccal tablets were excluded from the calculation because they are not defined in the guidelines [3, 7] and cannot be converted oral morphine equivalent dose.
All data analyses were carried out with SAS v.9.4, and P values of <0.05 were considered significant.
Results
Analysis population
The patient flow chart is shown in Fig. 2. From the database, 19,176 patients were eligible for the study, and of these, the opioid dose could be calculated in 19,107. There were 13,517 (70.7%) in the control, 111 (0.6%) in the case I, and 682 (3.5%) in the case II group. Supplement shows the distribution of opioid dose by group.
Factors associated with long-term, high-dose prescription of opioid
Table 1 shows the results of descriptive analysis of all factors examined as factors relevant to long-term, high-dose prescription, with detailed definitions of each factor provided in the Supplement. In the evaluation of patient background and cancer site and metastasis and comorbidities, there were significant differences in age, esophageal cancer, breast cancer, bone metastasis, distant metastasis, back pain, schizophrenia, emotional disorder, and neurological disorder in both comparisons between the control and case I groups and between the control and case II groups. In the evaluation of the period prior to start opioid prescription, duration from cancer diagnosis to start of opioid prescription, prescription of non-opioid analgesics, chemotherapy, and radiotherapy were significantly different in both comparisons. In the evaluation during opioid prescription, mean opioid dose and prescription of non-opioid analgesics, tricyclic antidepressant (TCA), selective serotonin reuptake inhibitor (SSRI), serotonin noradrenaline reuptake inhibitor (SNRI), gabapentinoids, anticonvulsants, and central muscle relaxants were significantly different in both comparisons.
Table 2 shows the explanatory variables included in the ordinal logistic regression analysis and the results of analysis. Among the factors that showed significant differences in both comparisons in the descriptive statistics, bone metastasis was excluded from the ordinal logistic analysis because it was considered to be related to distant metastasis, and schizophrenia, emotional disorder, and neurological disorder were also excluded because they were considered to be related to TCA, SSRI, and SNRI. In addition, of the factors that did not meet the criteria in step 1 for selecting variables, cancer site of large intestine, anus, and anal, osteoarthritis of knee, spinal stenosis, spondylosis, radiotherapy, and chemotherapy during opioid prescription were included as explanatory variables from a clinical perspective. In the patient background, back pain (OR 1.346, 95% CI 1.068–1.697) and distant metastasis (OR 1.802, 1.422–2.285) showed a significant association with long-term, high-dose opioid prescription. The variables evaluated during opioid prescription include mean dose of opioids (OR 1.022, 1.021–1.024), non-opioid analgesics (OR 8.157, 4.207–15.815), SSRI (OR 1.990, 1.045–3.472), SNRI (OR 2.432, 1.704–3.472), gabapentinoids (OR 4.595, 3.648–5.788), anticonvulsants (OR 2.804, 2.049–3.837), and chemotherapy (OR 2.050, 1.509–2.785) showed a significant association with long-term, high-dose opioid prescription.
Assessment of the rescue medication prescription
Table 3 shows the status of rescue drug prescription in each group. The percentage of patients receiving rescue medication was significantly higher in both case I group and case II group than the control group. In the analysis of patients receiving rescue medication, the mean number of rescue drug doses per day was significantly higher in case I group (1.25 times) and case II group (1.51 times) than in the control group (1.04 times). The mean rescue drug dose per day was significantly higher in case I group (32.23 mg) and case II group (43.62 mg) than in the control group (8.24 mg).
Discussion
The present study attempted to identify factors associated with long-term prescription of opioids at high-dose levels for cancer patients for the first time in Japan. As a result, there were 13,517 patients in the control group who were prescribed opioids for less than 183 days, while 111 and 682 patients in the case I and case II groups who were prescribed opioids for more than 183 days and averaged more than 120 mg/day, respectively. The majority of the study population fell into the control group, of which 70.1% averaged less than 30 mg/day, while about 4% of the study population was identified as being on long-term, high-dose prescriptions. In addition, distant metastasis, prescription of non-opioid analgesics, SSRI, SNRI, gabapentinoids, and anticonvulsants during the opioid prescription period, chemotherapy, and back pain were shown to be associated with long-term, high-dose opioid prescribing.
In the present study, although esophageal cancer, breast cancer, and colorectal cancer were considered as candidate factors associated with long-term, high-dose prescription of opioids based on descriptive statistics results, no significant association was found in the ordinal logistic regression analysis. Although cancer prognosis varies by race and environmental factors and is not generally comparable, several studies described the relationship between cancer type and long-term opioid prescription. A study by Jones et al. reviewing studies of long-term prescription of opioid in cancer survivors noted a high rate of long-term opioid therapy in head and neck cancer [4]. The head and neck cancer was not identified as an associated factor in the present study; Jones et al. included all cancer survivors, whereas the present study included only patients who were prescribed opioid, which might bring different results due to population differences from the present study. The study by Salz et al. found that chronic use of opioids among colorectal and lung cancer survivors exceeded chronic use among controls [10]. In the present study, the point estimate for colorectal cancer also showed an association with long-term use. However, there are limitations in comparing the results of the present study with those of Salz et al. because they are comparisons with controls in non-cancer patients. Since there are limited reports on cancer types, further studies are needed.
Patients with distant metastasis are more likely to have severe cancer, which is consistent with the report on opioid use in colorectal cancer patients by Chen et al. [11] that the more severe the stage, the longer the duration of opioid prescription.
Regarding the prescription of non-opioid analgesics, Murphy et al. reported an association between polypharmacy and long-term prescription of opioids [12], and it is possible that pain management using a combination of analgesics is being implemented in clinical practice. In addition, Desai et al. reported that mental health comorbidity increased the risk of opioid prescription in older breast cancer patients [13], and the association with tranquilizers has also been reported in a study of non-cancer patients by Hauser et al. [14]. Shah et al. also reported that patients with chemotherapy-induced peripheral neuropathy were associated with long-term opioid prescription [15]. The results of the present study are consistent with previous reports that SSRI and SNRI as antidepressants, gabapentinoids, and antiepileptic drugs that are sometimes used to treat neuropathic pain, and chemotherapy were shown to be factors associated with long-term, high-dose of opioids.
We were the first to examine back pain as a factor in long-term, high-dose opioid prescriptions and showed a significant association. The reasons that back pain was identified as a factor are considered to be as follows. Firstly, since patients with bone metastasis were also included in this study, it is possible that pain associated with bone metastasis was diagnosed as back pain. The percentage of patients with bone metastasis among those diagnosed with back pain was higher in case I (44.0%) and case II (42.6%) groups than in the control group (20.8%). Secondly, opioids might have been prescribed for non-cancer pain. When we confirmed the details of the diagnostic names related to back pain, the 90% patients in both the case and control groups were diagnosed with lower back pain (Supplement). Nakamura et al. also reported that about 15.4% of Japanese people had chronic musculoskeletal pain, of which 65% had lower back pain, a high percentage [16]. As advances in cancer treatment have improved survival rates for many types of cancer, the patients complaining of non-cancer chronic pain are likely to increase. In Japan, the use of opioids for non-cancer chronic pain is likely to be prolonged, and problems such as side effects of opioids could occur due to excessive pursuit of pain relief; therefore, appropriate use is important. However, opioids might actually be prescribed without sufficiently distinguishing between these types of pain [3].
We further exploratory evaluated the status of rescue medications. The percentage of patients receiving both periodical medication and rescue medication was higher in the case groups than in the control group, and the rescue drug dosing frequency and level per day were also higher in the case groups. Patients with long-term, high-dose prescription of opioid were shown to have a higher frequency of use and prescription of rescue medications; however, confounding factors could not be ruled out because patients background were not matched; thus, the result needs careful interpretation.
The present study using a large-scale, hospital-based administrative claims database involves limitations arising from database characteristics. First, the database employed for this study covered only the data at hospitals having introduced an acute inpatient care system. Because this database does not track data when patients visit to other hospitals, if opioids were prescribed at other hospitals, the dose and frequency of opioid administration may have been underestimated. Second, the opioid dose might be underestimated because fentanyl sublingual tablets and fentanyl buccal tablets are not reflected in the prescription dose. However, since most drugs can be converted to morphine, we believe that this issue will have a small impact on the results. Third, because the data from this database are based on payment-related information, it is not possible to capture whether the patient actually took the drug. Fourth, since we were unable to assess the severity and type of pain, we cannot discuss the clinical appropriateness of the opioid prescription.
Conclusions
We investigated the situation of cancer patients prescribed opioids and the factors leading to long-term, high-dose prescription. Within the scope of the study using the claims data, it was found that about 4% of cancer patients prescribed opioids in Japan were prescribed long term and high doses. Some of the factors identified were similar to those previously reported; however, the study also found new risks, such as back pain. This suggests the potential existence of a situation in Japan where opioids are used in cancer patients with insufficient distinction between cancer pain and non-cancer chronic pain. In order to avoid the careless use of opioids, it is essential to be careful about comorbidities in cancer patients, to evaluate the pain in individual patients for distinction between these types of pain and to select analgesics including opioids tailored to individual patients.
Data availability
The data that support the findings of this study are available from Medical Data Vision Co., Ltd, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Medical Data Vision Co., Ltd.
Code availability
Code sharing not applicable to this article as some or all code used during the study were provided by a third party.
References
Monitoring of Cancer Incidence in Japan - Survival 2009-2011 Report (Center for Cancer Control and Information Services, National Cancer Center, 2020) [Japanese]. Available from https://ganjoho.jp/reg_stat/statistics/data/dl/en.html#anchor3. Accessed 1 June 2021
Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T, Research Group of Population-Based Cancer Registries of Japan Comparative Study (2011) Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 41:40-51. https://doi.org/10.1093/jjco/hyq167
The Japanese Society of Pain Clinicians (2016) Guidelines for pharmacologic management of neuropathic pain, 2nd edn. Shinko Trading Press, Tokyo
Jones KF, Fu MR, Merlin JS, Paice JA, Bernacki R, Lee C, Wood LJ (2021) Exploring factors associated with long-term opioid therapy in cancer survivors: an integrative review. J Pain Symptom Manage 61:395–415. https://doi.org/10.1016/j.jpainsymman.2020.08.015
Azuma K, Abe H, Hozumi J et al (2020) Prefectural adequacy of opioid availability for cancer pain and its determinants in Japan: a preliminary study. JMA J 3:340-346. 10.31662/jmaj.2020-0037
Medical Data Vision Co., Ltd. Available from https://en.mdv.co.jp/about-mdv-database/. Accessed 1 June 2021
Japanese Society for Palliative Medicine Secretariat (2020) Clinical guidelines for cancer pain management, 3rd edn. Kanehara, Tokyo
Häuser W, Bock F, Engeser P, Tölle T, Willweber-Strumpfe A, Petzke F (2014) Long-term opioid use in non-cancer pain. Dtsch Arztebl Int 111:732–740. https://doi.org/10.3238/arztebl.2014.0732
Ministry of Health, Labour and Welfare. Guidance on the appropriate use of medical narcotics. Available from https://www.mhlw.go.jp/bunya/iyakuhin/yakubuturanyou/other/iryo_tekisei_guide.html. Accessed 1 June 2021
Salz T, Lavery JA, Lipitz-Snyderman AN, Boudreau DM, Moryl N, Gillespie EF, Korenstein D (2019) Trends in opioid use among older survivors of colorectal, lung, and breast cancers. J Clin Oncol 37:1001–1011. https://doi.org/10.1200/jco.18.00938
Chen L, Chubak J, Yu O et al (2019) Changes in use of opioid therapy after colon cancer diagnosis: a population-based study. Cancer Causes Control 30:1341–1350. https://doi.org/10.1007/s10552-019-01236-5
Murphy CC, Fullington HM, Alvarez CA, Betts AC, Lee SJC, Haggstrom DA, Halm EA (2018) Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer 124:2850–2857. https://doi.org/10.1002/cncr.31389
Desai R, Camacho F, Tan X, LeBaron V, Blackhall L, Balkrishnan R (2019) Mental health comorbidities and elevated risk of opioid use in elderly breast cancer survivors using adjuvant endocrine treatments. J Oncol Pract 15:e777–e786. https://doi.org/10.1200/jop.18.00781
Häuser W, Schubert T, Scherbaum N, Tölle T (2018) Guideline-recommended vs high-dose long-term opioid therapy for chronic noncancer pain is associated with better health outcomes: data from a representative sample of the German population. Pain 159:85–91. https://doi.org/10.1097/j.pain.0000000000001067
Shah A, Hoffman EM, Mauermann ML, Loprinzi CL, Windebank AJ, Klein CJ, Staff NP (2018) Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry 89:636–641. https://doi.org/10.1136/jnnp-2017-317215
Nakamura M, Nishiwaki Y, Ushida T, Toyama Y (2011) Prevalence and characteristics of chronic musculoskeletal pain in Japan. J Orthop Sci 16:424–432. https://doi.org/10.1007/s00776-011-0102-y
Acknowledgements
The authors would like to thank Ms. Manami Yoshida of Shionogi & Co., Ltd., for her planning of the study, including development of the protocol, and interpretation of the data, and Mr. Hidetoshi Shibahara and Mr. Kouzaburo Kobayashi of CRECON Medical Assessment, Inc., on data management and statistical analysis.
Funding
This study was funded by Shionogi & Co., Ltd.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis planning were performed by Hirokazu Mishima, Chika Sakai, and Yuichi Koretaka. Analysis of the data was performed by Hirokazu Mishima and Yuichi Koretaka, and all authors participated in consideration on validity of analysis methods and interpretation of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Tatsuya Hashimoto received honoraria from Daiichi Sankyo, Eisai, Hisamitsu Pharmaceutical, and Pfizer.
Hirokazu Mishima and Yuichi Koretaka are employees of Shionogi.
Chika Sakai was an employee of Shionogi at the time the study was conducted. Chika Sakai is currently affiliated with Janssen Pharmaceutical K.K., Tokyo, Japan.
Yoji Saito received honoraria from Asahi Kasei Pharma, AYUMI Pharmaceutical, Daiichi Sankyo, Edwards Lifesciences, GlaxoSmithKline, Hisamitsu Pharmaceutical, Japan Blood Products Organization, Kyowa Kirin, Maruishi Pharmaceutical, Nihon Kohden, Nippon Zoki Pharmaceutical, Pfizer, Terumo, and Tsumura.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 189 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashimoto, T., Mishima, H., Sakai, C. et al. An exploratory study of factors associated with long-term, high-dose opioid prescription in cancer patients in Japan based on a medical claims database. Support Care Cancer 30, 6879–6888 (2022). https://doi.org/10.1007/s00520-022-07121-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07121-3