Abstract
Background
Skin toxicity in patients affected by metastatic colorectal cancer (mCRC) treated with epidermal growth factor receptor (EGFR) inhibitors is well known. However, ad hoc ESMO guidelines have only recently been published.
Aim and methods
To describe the management (pre-emptive or reactive) of anti-EGFR-related cutaneous adverse events (AEs), in a real-life clinical context, in a selected population of patients with left-sided, metastatic RAS/BRAF wild-type mCRC treated with doublet chemotherapy plus anti-EGFR monoclonal antibody (i.e., panitumumab or cetuximab) as first-line regimen at 22 Institutions. The measured clinical outcomes were treatment-related adverse events, objective response rate (ORR), progression-free survival (PFS), and overall survival (OS).
Results
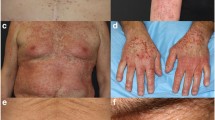
Of 515 patients included in the analysis, 173 (33.6%) received a pre-emptive and 342 (66.4%) a reactive treatment. The median follow-up period for the overall population was 30.0 months. A significantly lower incidence of any grade acneiform rash was found in the pre-emptive compared to the reactive cohort both in the overall population (78.6% vs 94.4%, p < 0.001) and in patients treated with panitumumab (76.1% vs 93.7%, p < 0.001) or cetuximab (83.3% vs 95.4%, p = 0.004), respectively. A lower incidence of any grade (41.6% vs 50.9%, p = 0.047) but a higher incidence of G3-G4 (9.2% vs 4.7%, p = 0.042) paronychia/nail disorders were found in the pre-emptive compared to the reactive cohort. Nevertheless, a lower rate of patients within the reactive compared to the pre-emptive cohort was referred to dermatological counseling (21.4% vs 15.3%, respectively, p = 0.001). A higher rate of anti-EGFR therapy modification was needed in the pre-emptive compared to the reactive cohort (35.9% vs 41.6%, respectively, p < 0.001). The pre-emptive approach did not reduce the efficacy of antineoplastic therapy compared to the reactive in terms of ORR (69.2% vs 72.8%), median PFS (12.3 vs 13.0 months), and median OS (28.8 vs 33.5 months).
Conclusion
Although recommended by international guidelines, the pre-emptive approach of anti-EGFR-related skin toxicity in mCRC patients still appears less adopted in daily clinical practice, compared to the reactive one. A wider reception and application of this indication is desirable to improve patients’ quality of life without compromising the continuity and efficacy of antineoplastic therapy.


Similar content being viewed by others
Data sharing
The dataset used during the present study are available from the corresponding author upon reasonable request.
References
Van Cutsem E, Cervantes A, Adam R et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422. https://doi.org/10.1093/annonc/mdw235
Linee guida AIOM TUMORI DEL COLON – Edizione 2020. Available at https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Colon.pdf. Accessed 2021 July
Parisi A, Giampiero P, Cannita K et al (2021, ISSN 1533-0028) Clinicians’ attitude to doublet plus anti-EGFR versus triplet plus bevacizumab as first line treatment in left-sided RAS and BRAF wild-type metastatic colorectal cancer patients: a multicentre, “real-life”, case-control study. Clin Colorectal Cancer. https://doi.org/10.1016/j.clcc.2021.07.003
Grassadonia A, Di Marino P, Ficorella C et al (2019) Impact of primary tumor location in patients with RAS wild-type metastatic colon cancer treated with first-line chemotherapy plus anti-EGFR or anti-VEGF monoclonal antibodies: a retrospective multicenter study. J Cancer 10(24):5926–5934. https://doi.org/10.7150/jca.34550
Parisi A, Cortellini A, Venditti O et al (2021) Post-induction management in patients with left-sided RAS and BRAF wild- type metastatic colorectal cancer treated with first-line anti-EGFR-based doublet regimens: a multicentre study. Front Oncol. https://doi.org/10.3389/fonc.2021.712053
Parisi A, Cortellini A, Cannita K et al (2020) Evaluation of second-line anti-VEGF after first-line anti-EGFR based therapy in RAS wild-type metastatic colorectal cancer: the multicenter “SLAVE” study. Cancers (Basel) 12(5):1259. https://doi.org/10.3390/cancers12051259
Lacouture ME (2006) Mechanism of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 6:803–812
Fabbrocini G, Panariello L, Caro G et al (2015) Acneiform rash induced by EGFR inhibitors: review of the literature and new insights. Skin Appendage Disord 1(1):31–37
Lichtenberger BM, Gerber PA, Holcmann M et al (2013) Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med 5(199):199ra111
Price TJ, Peeters M, Kim TW et al (2014) Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 15(6):569–579
Lacouture ME, Anadkat MJ, Bensadoun RJ et al (2011) Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 19(8):1079–1095
Garden BC, Wu S, Lacouture ME (2012) The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol 67(3):400–408
Lacouture ME, Sibaud V, Gerber PA et al (2021) Prevention and management of dermatological toxicities related to anticancer agents: ESMO Clinical Practice Guidelines. Ann Oncol 32(2):157–170
Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, Xu F, Yassine M (2010) Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 28(8):1351–1357
Kobayashi Y, Komatsu Y, Yuki S, Fukushima H, Sasaki T, Iwanaga I, Uebayashi M, Okuda H, Kusumi T, Miyagishima T, Sogabe S, Tateyama M, Hatanaka K, Tsuji Y, Nakamura M, Konno J, Yamamoto F, Onodera M, Iwai K et al (2015) Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study. J-STEPP Future Oncol 11(4):617–627
Dascalu B, Kennecke HF, Lim HJ, Renouf DJ, Ruan JY, Chang JT, Cheung WY (2016) Prophylactic versus reactive treatment of acneiform skin rashes from epidermal growth factor receptor inhibitors in metastatic colorectal cancer. Support Care Cancer 24(2):799–805
Bouché O, Ben Abdelghani M, Labourey JL et al (2019) Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol 25(29):4007–4018
Kafatos G, Dube S, Burdon P, Demonty G, Flinois A, Leclerc M, Lowe K, Feudjo-Tepie M, Segaert S (2020) Management of EGFR inhibitor-induced skin toxicity and factors impacting patients’ adherence to skin toxicity treatment: health care provider and patient surveys in European Oncology Centers. Clin Colorectal Cancer 19(2):100–108.e9
Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E (2018) Dermatologic toxicity occurring during anti-EGFR monoclonal inhibitor therapy in patients with metastatic colorectal cancer: a systematic review. Clin Colorectal Cancer 17(2):85–96
Scope A, Agero AL, Dusza SW, Myskowski PL, Lieb JA, Saltz L, Kemeny NE, Halpern AC (2007) Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol 25(34):5390–5396
Yamada M, Iihara H, Fujii H, Ishihara M, Matsuhashi N, Takahashi T, Yoshida K, Itoh Y (2015) Prophylactic effect of oral minocycline in combination with topical steroid and skin care against panitumumab-induced acneiform rash in metastatic colorectal cancer patients. Anticancer Res 35(11):6175–6181
Petrelli F, Borgonovo K, Barni S (2013) The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol 8:173–181
Dienstmann R, Braña I, Rodon J, Tabernero J (2011) Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 16(12):1729–1740
Peeters M, Siena S, Van Cutsem E, Sobrero A, Hendlisz A, Cascinu S, Kalofonos H, Devercelli G, Wolf M, Amado RG (2009) Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer. 115(7):1544–1554
De Tursi M, Zilli M, Carella C, Auriemma M, Lisco MN, Di Nicola M, Di Martino G, Natoli C, Amerio P (2017) Skin toxicity evaluation in patients treated with cetuximab for metastatic colorectal cancer: a new tool for more accurate comprehension of quality of life impacts. Onco Targets Ther 10:3007–3015. https://doi.org/10.2147/OTT.S127795
Author information
Authors and Affiliations
Contributions
All the authors contributed to the publication according to the ICMJE guidelines for the authorship. All the authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical statement
All patients alive at the time of data collection provided informed consent to participate to this retrospective observational non-interventional study. The procedures followed were in accordance with the precepts of Good Clinical Practice and the declaration of Helsinki. The study was approved by the respective local ethical committees on human experimentation of each institution, after previous approval by the coordinating center (Comitato Etico delle Province di L’Aquila e Teramo, protocol number 21, approved on July 16, 2020).
Informed consent
All patients alive at the time of data collection provided an informed consent to participate to this retrospective observational non-interventional study.
Conflict of interest
A.P. reported receiving advisory board fees from GSK. A.C. reported receiving consulting/advisory board fees from Astrazeneca, MSD, Roche and BMS; speakers’ fee from Novartis, Astellas, MSD, Astrazeneca. A.P. reported receiving fees from Eli-Lilly, MSD and Servier. R.G. reported receiving fees from Amgen and Servier and advisory board fees from Amgen, Servier, Bayer and Merck-Serono. GS reported receiving advisory board/Editorial fees from: Novartis, Servier, Teva, Bayer, Genetic, Epionpharma.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Antonetti, P., Fargnoli, M.C., Porzio, G. et al. A multicenter study of skin toxicity management in patients with left-sided, RAS/BRAF wild-type metastatic colorectal cancer treated with first-line anti-EGFR-based doublet regimen: is there room for improvement?. Support Care Cancer 30, 2455–2465 (2022). https://doi.org/10.1007/s00520-021-06652-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06652-5