Abstract
Introduction
Venetoclax along with hypomethylating agents (HMAs) is the new standard therapy for older patients with acute myeloid leukemia (AML) not fit for intensive frontline induction chemotherapy. Venetoclax is associated with fatal episodes of tumor lysis syndrome (TLS) in chronic lymphocytic leukemia (CLL), and recommendations are for its initiation for CLL and AML in the inpatient setting with close monitoring. Herein, we evaluated the safety of outpatient venetoclax ramp up when given in addition to HMAs for the treatment of AML.
Methods
We conducted a retrospective review of patients diagnosed with AML at our institution from 12/1/2016 until 7/1/2020. We identified patients who received HMAs and venetoclax for AML, either as frontline or relapsed/refractory therapy. Records were reviewed for evidence of laboratory or clinical tumor lysis episodes in all patients.
Results
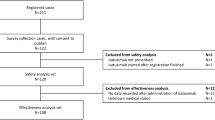
Between 12/1/2016 and 7/1/2020 43, patients at our institution received venetoclax/HMA for the treatment of AML. Thirty-nine patients (91%) had venetoclax initiation and ramp up in the outpatient setting. One episode of laboratory TLS (2.5%) was identified. This patient required admission to the hospital for rasburicase and IV fluids with resolution of the laboratory effects without resultant clinical TLS. There were no episodes of clinical TLS in either group. Thirty-day mortality from venetoclax initiation was 0% in both groups.
Conclusion
Our experience with HMAs and venetoclax showed that outpatient ramp up of venetoclax is safe with a very low risk of laboratory TLS (2.5%) and no evidence of clinical TLS within our cohort.
Similar content being viewed by others
Data availability
Not applicable
Code availability
Not applicable
References
Grimwade WH, D, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A (1998) The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood 92(7):2323–2333
"National Cancer Institute Surveillance, Epidemiology, and End Results Program Cancer Stat Facts: Leukemia–Acute Myeloid Leukemia (AML) Vol 2020."
Sekeres MA et al (2020) American Society of Hematology 2020 Guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv 4(15):3528–3549
Fenaux P et al (2010) Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 28(4):562–569
Dombret H et al (2015) International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126(3):291–299
DiNardo PK, CD, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A (2019) Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133(1):7–17
DiNardo CD et al (2020) Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383(7):617–629
"National Comprehensive Cancer Network. Acute Myeloid Leukemia (V3.2020)."
Vo TT et al (2012) Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151(2):344–355
Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, Anderson MA, Beaven AW, Rosen ST, Tam CS, Prine B, Agarwal SK, Munasinghe W, Zhu M, Lash LL, Desai M, Cerri E, Verdugo M, Kim SY, Humerickhouse RA, Gordon GB, Kipps TJ, Roberts AW (2017) Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. The Lancet Oncology 18(2):230–240
Roberts AW et al (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374(4):311–322
"U.S. Food and Drug Administration. Highlights of Prescribing Information: Venclexta; 2018."
Jonas BA, Pollyea DA (Dec 2019) How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia 33(12):2795–2804
Howard JD, S, Pui CH (2011) The tumor lysis syndrome. N Engl J Med 364:1844–1854
Roeker LE et al (2019) Tumor lysis, adverse events, and dose adjustments in 297 venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res 25(14):4264–4270
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of the work, acquisition, analysis, or interpretation of data, drafting the work or revising it critically for important intellectual content, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Rhode Island Hospital approved this study.
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pelcovits, A., Moore, J., Bakow, B. et al. Tumor lysis syndrome risk in outpatient versus inpatient administration of venetoclax and hypomethlators for acute myeloid leukemia. Support Care Cancer 29, 5323–5327 (2021). https://doi.org/10.1007/s00520-021-06119-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06119-7