Abstract
Objective
The aim of this meta-analysis was to evaluate the effectiveness of glutamine for preventing or treating moderate-to-severe oral mucositis induced by chemotherapy or radiation therapy in patients with cancer.
Methods
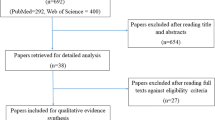
PubMed, Cochrane Library, and Embase were searched for eligible randomized controlled trials (RCTs) up to June 2020. The outcomes analyzed were oral mucositis (at all levels of severity). Data were pooled using the random-effects model and are expressed as risk ratios (RRs) and corresponding 95% confidence intervals (CIs). Heterogeneity was assessed and quantified using I2.
Results
Sixteen RCTs were included in this review. In this meta-analysis, compared with placebo, glutamine significantly reduced the incidence of grade 3 and 4 oral mucositis induced by chemotherapy or radiation therapy (RR, 0.53; 95% CI, 0.32–0.88). In subgroup analysis, oral glutamine administration (RR, 0.56; 95% CI, 0.34–0.92) and a medium or low daily dose of glutamine (RR, 0.58; 95% CI, 0.44–0.77; RR, 0.53; 95% CI, 0.28–0.94; respectively) decreased risk. Glutamine caused a borderline significant reduction in the risk of grade 3 and 4 oral mucositis induced by radiotherapy (RR, 0.75; 95% CI, 0.58–0.99) and especially in its prevention (RR, 0.51; 95% CI, 0.28–0.94).
Conclusions
Glutamine significantly reduces the risk of oral mucositis during chemotherapy or radiation therapy. Furthermore, large prospective trials are required to support these findings.





Similar content being viewed by others
Change history
13 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00520-021-06112-0
References
Forastiere AA, Trotti A (1999) Radiotherapy and concurrent chemotherapy: a strategy that improves locoregional control and survival in oropharyngeal cancer. J Natl Cancer Inst 91(24):2065–2066
Seiwert TY, Cohen EE (2005) State-of-the-art management of locally advanced head and neck cancer. Br J Cancer 92(8):1341–1348
Sonis ST, Elting LS, Keefe DM et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025
Merlano M, Marchetti G (2003) Radiochemotherapy in head and neck cancer. Cancer Treat Rev 29:291–296
Brizel D, Albers M, Fischer S et al (1998) Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 338:1798–1804
Trotti A, Bellm L, Epstein J et al (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66:253–262
Peterson DE, Boers-Doets CB, Bensadoun RJ, Herrstedt J (2015) ESMO Guidelines Committee. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 26(supl 5):v139–v151
Lalla RV, Sonis ST, Peterson DE (2008) Management of oral mucositis in patients who have cancer. Dent Clin N Am 52(1):61–77 viii
Skubitz KM, Anderson PM (1996) Oral glutamine to prevent chemotherapy induced stomatitis: a pilot study. J Lab Clin Med 127(2):223–228
Pathak S, Soni TP, Sharma LM, Patni N, Gupta AK (2019) A randomized controlled trial to evaluate the role and efficacy of oral glutamine in the treatment of vhemo-radiotherapy-induced oral mucositis and dysphagia in patients with oropharynx and larynx carcinoma. Cureus 11(6):e4855
Silverman S Jr (2007) Diagnosis and management of oral mucositis. J Support Oncol 5(2 Suppl 1):13–21
Fox AD, Kripke SA, De Paula J, Berman JM, Settle RG, Rombeau JL (1988) Effect of a glutamine supplemented enteral diet on methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr 12:325–331
Leung HW, Chan AL (2016) Glutamine in alleviation of radiation-induced severe oral mucositis: a meta-analysis. Nutr Cancer 68(5):734–742
de Menêses AG, Normando AGC, Porto de Toledo I, Reis PED, Guerra ENS (2019, 2019) Effects of oral supplementation in the management of oral mucositis in cancer patients: a meta-analysis of randomized clinical trials. J Oral Pathol Med. https://doi.org/10.1111/jop.12901
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Int J Surg 8(5):336–341
Higgins JP, Green S (2008) Guide to the contents of a Cochrane protocol and review. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Wiley-Blackwell, Hoboken, p 51–79
Cox JD, Stetz J, Pajak TF (1995) Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
Organization WH (1979) WHO handbook for reporting results of cancer treatment. World Health Organization: WHO offset publication, Geneva, 48
National Cancer Institute (2017) Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. European Organization for Research and Treatment of Cancer
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Higgins J, Deeks JJ, Altman DG (2008) Special topics in statistics. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Wiley-Blackwell, Hoboken, 48–529
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Manzi NM, Silveira RCC, Reis PED (2015) Prophylaxis for mucositis by ambulatory chemotherapy: systematic review. J Adv Nurs 72(4):735–746
Khan SA, Wingard JR (2001) Infection and mucosal injury in cancer treatment. J Natl Cancer Inst Monogr (29):31–36
Shabert JK, Winslow C, Lacey JM, Wilmore DW (1999) Glutamine-antioxidant supplementation increases body cell mass in AIDS patients with weight loss: a randomized, double-blind controlled trial. Nutrition 15(11):860–864
Haussinger D (1998) Hepatic glutamine transport and metabolism. Adv Enzymol Relat Areas Mol Biol 72:43–86
Lacey JM, Wilmore DW (1990) Is glutamine a conditionally essential amino acid? Nutr Rev 48(8):297–309
Okuno SH, Woodhouse CO, Loprinzi CL et al (1999) Phase III controlled evaluation of glutamine for decreasing stomatitis in patients receiving fluorouracil (5-FU)-based chemotherapy. Am J Clin Oncol 22(3):258–261
Nihei S, Sato J, Komatsu H et al (2018) The efficacy of sodium azulene sulfonate L-glutaminefor managing chemotherapy-induced oral mucositis in cancer patients: a prospective comparative study. J Pharm Health Care Sci 4:20
Huang CJ, Huang MY, Fang PT et al (2019) Randomized double-blind, placebo-controlled trial evaluating oral glutamine on radiation-induced oral mucositis and dermatitis in head and neck cancer patients. Am J Clin Nutr 109(3):606–614
Pattanayak L, Panda N, Dash MK, Mohanty S, Samantaray S (2016) Management of chemoradiation-induced mucositis in head and neck cancers with oral glutamine. J Glob Oncol 2(4):200–206
Giris M, Erbil Y, Oztezcan S et al (2006) The effect of heme oxygenase-1 induction by glutamine on radiation-induced intestinal damage: the effect of heme oxygenase-1 on radiation enteritis. Am J Surg 191:503–509
Ersin S, Tuncyurek P, Esassolak M, Alkanat M, Buke C, Yilmaz M, Telefoncu A, Kose T (2000) The prophylactic and therapeutic effects of glutamine- and arginine-enriched diets on radiation-induced enteritis in rats. J Surg Res 89:121–125
Coghlin Dickson TM, Wong RM, Offrin RS et al (2000) Effect of oral glutamine supplementation during bone marrow transplantation. J Parenter Enter Nutr 24(2):61–66
Tsujimoto T, Yamamoto Y, Wasa M et al (2015) L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: a double-blind, randomized, placebo- controlled trial. Oncol Rep 33(1):33–39
Lopez-Vaquero D, Gutierrez-Bayard L, Rodriguez-Ruiz JA, Saldaña-Valderas M, Infante-Cossio P (2017) Double-blind randomized study of oral glutamine on the management of radio/chemotherapy-induced mucositis and dermatitis in head and neck cancer. Mol Clin Oncol 6(6):931–936
Tanaka Y, Takahashi T, Yamaguchi K, Osada S, Shimokawa T, Yoshida K (2016) Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: a feasibility study. Support Care Cancer 24(2):933–941
Huang EY, Leung SW, Wang CJ et al (2000) Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys 46(3):535–539
Cerchietti LC, Navigante AH, Lutteral MA et al (2006) Double-blinded, placebo-controlled trial on intravenous L-alanyl-L-glutamine in the incidence of oral mucositis following chemoradiotherapyin patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 65(5):1330–1337
Choi K, Lee SS, Oh SJ et al (2007) The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin Nutr 26(1):57–62
Peterson DE, Jones JB, Petit RG (2007) Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer 109(2):322–331
Jebb SA, Osborne RJ, Maughan TS, Mohideen N, Mack P, Mort D, Shelley MD, Elia M (1994) 5-Fluorouracil and folimc acid-induced mucositis: no effect of oral glutamine supplementation. Br J Cancer 70(4):732–735
Diwan AK, Khan S (2018) Assessing role of oral glutamine supplementation in radiation induced oral mucositis in head and neck cancers. Ann Int Med Dental Res 4(2):1–6
Availability of data and material
All data, models, and code generated or used during the study appear in the submitted article.
Code availability
Software application.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.-R.P. and H.-H.L.; methodology, T.-R.P.; software, T.-R.P.; validation, T.-R.P., L.-J.Y., and H.-H.L.; formal analysis, T.-R.P. and L.-J.Y.; investigation, H.-H.L.; data curation, L.-J.Y.; writing—original draft preparation, T.-R.P.; writing—review and editing, H.-H.L. and T.-W.W. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Waivers and N/A.
Consent for publication
All authors agree to publish.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. ESM is updated.
Supplementary Information
ESM 1
(DOCX 19.9 kb)
Rights and permissions
About this article
Cite this article
Peng, TR., Lin, HH., Yang, LJ. et al. Effectiveness of glutamine in the management of oral mucositis in cancer patients: a meta-analysis of randomized controlled trials. Support Care Cancer 29, 4885–4892 (2021). https://doi.org/10.1007/s00520-021-06060-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06060-9