Abstract
Background
Selecting study endpoints in prospective cancer cachexia trials remains poorly defined. The aim of this study was to further evaluate associations in changes in weight, body composition, functional outcomes, and patient-reported outcomes (PROs) in patients with metastatic cancer.
Methods
We completed a 2-year (2016–2018) observational study in patients with metastatic solid cancer and ECOG performance status 0 to 2 while receiving chemotherapy and/or immunotherapy. We completed assessments at study enrollment and 3 months from enrollment. We analyzed longitudinal changes in weight and body composition using validated methods. Functional assessments included the 6-Min Walk Test, Timed Up and Go Test, and Short Physical Performance Battery. PROs included the Functional Assessment of Anorexia/Cachexia Therapy and Functional Assessment of Cancer Therapy Fatigue. We analyzed changes in body composition and functional assessment using paired t tests. Additionally, we utilized linear regression models to assess relationships between changes in body composition and function outcomes and PROs, adjusting for age and sex.
Results
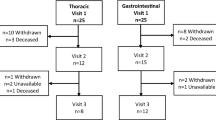
A total of 57 patients completed baseline assessments, but 19 patients did not complete 3-month assessments (5 died, 1 hospice, 13 withdrew). Of the 38 patients with complete data, the mean age was 61.8 years and 47% were female. Metastatic cancer types included 71% gastrointestinal, 13% lung, and 8% gynecologic. Half received chemotherapy, 16% immunotherapy, and 34% a combination. From enrollment to 3 months, we did not observe a change in weight or skeletal muscle but did find an increase in total adipose tissue (16.9 ± 52.4 cm2, 95% CI − 33.79–0.63; p = 0.059; ~ 1.5 pounds). We did not observe any association with changes in weight with any functional outcomes or PROs. However, greater losses in skeletal muscle were associated with greater declines in physical function (6-Min Walk Test [B = 0.04, p = 0.01], Short Physical Performance Battery [B = 2.44, p < 0.01]).
Conclusions
Patients with metastatic cancer receiving cancer-directed therapy may not experience a change in body weight. However, we found an association between losses in skeletal muscle and greater declines in physical function. Therefore, when selecting study endpoints, prospective cancer cachexia studies may consider selecting changes in body composition over weight.
Similar content being viewed by others
References
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA et al (2016) Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34(12):1339–1344
Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ (2016) Cancer cachexia: beyond weight loss. J Oncol Pract 12(11):1163–1171
Tisdale MJ (2003) Pathogenesis of cancer cachexia. J Support Oncol 1(3):159–168
Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A (2013) Cancer cachexia--pathophysiology and management. J Gastroenterol 48(5):574–594
Stewart GD, Skipworth RJ, Fearon KC (2006) Cancer cachexia and fatigue. Clin Med (Lond) 6(2):140–143
Maddocks M, Byrne A, Johnson CD, Wilson RH, Fearon KC, Wilcock A (2010) Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Support Care Cancer 18(12):1539–1544
Nipp RD, Fuchs G, El-Jawahri A et al (2018) Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist 23(1):97–104
Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Buchler MW, Friess H, Martignoni ME (2008) Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 12(7):1193–1201
Joglekar S, Nau PN, Mezhir JJ (2015) The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol 112(5):503–509
Utech AE, Tadros EM, Hayes TG, Garcia JM (2012) Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle 3(4):245–251
Ma JD, Heavey SF, Revta C, Roeland EJ (2014) Novel investigational biologics for the treatment of cancer cachexia. Expert Opin Biol Ther 14(8):1113–1120
Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC (2016) Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 17(4):519–531
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 97(6):2333–2338
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006
Kasymjanova G, Correa JA, Kreisman H, Dajczman E, Pepe C, Dobson S, Lajeunesse L, Sharma R, Small D (2009) Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol 4(5):602–607
Podsiadlo D, Richardson S (1991) The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39(2):142–148
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):M85–M94
Blauwhoff-Buskermolen S, Ruijgrok C, Ostelo RW, de Vet HCW, Verheul HMW, de van der Schueren MAE, Langius JAE (2016) The assessment of anorexia in patients with cancer: cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer 24(2):661–666
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E (1997) Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manag 13(2):63–74
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Roeland E, Nelson S, Campillo A, et al. Inclusion criteria for cancer cachexia clinical trials: CT-defined skeletal muscle loss versus body weight loss. J Clin Oncol (Meeting Abstracts). 2015(suppl 29S; abstract 67)
Lee H, Troschel FM, Tajmir S et al (2017) Pixel-level deep segmentation: artificial intelligence quantifies muscle on computed tomography for body morphometric analysis. J Digit Imaging 30(4):487–498
Bridge CP, Rosenthal M, Wright B et al (2018) Fully-automated analysis of body composition from CT in cancer patients using convolutional neural networks. In: OR 2.0 Context-Aware Operating Theaters, Computer Assisted Robotic Endoscopy, Clinical Image-Based Procedures, and Skin Image Analysis. Springer, Berlin, pp 204–213
Funding
Dr. Roeland’s research is supported by the Cambia Health Foundation Sojourns Scholars Leadership Program, Alliance Cancer Control Program Junior Faculty Award (#UG1CA189823), and UC San Diego Clinical Translational Research Institute KL2 Career Development Award (#KL2TR001444).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Roeland is a consultant for the BASF, Napo Pharmaceuticals, Imuneering, Asahi Kasei Pharma, Prime Oncology, and American Imaging Management. He also has served on advisory boards for Heron Therapeutics and Vector Oncology. He also serves on the Data Safety Monitoring Board for the Galera Therapeutics, Oragenics Inc., and Enzychem Lifesciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roeland, E.J., Phull, H., Hagmann, C. et al. FIT: Functional and imaging testing for patients with metastatic cancer. Support Care Cancer 29, 2771–2775 (2021). https://doi.org/10.1007/s00520-020-05730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05730-4