Abstract
Purpose
Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting side-effect of neurotoxic cancer treatment impacting on long-term quality of life. Symptoms include numbness, tingling, and pain, affecting the distal extremities. However, patients often report symptoms discrepant from the expected symmetrical distribution and the degree of concurrence with objective assessment remains ill-defined. This study aimed to investigate severity and symmetry of neuropathy symptoms to enable comparison of objective measures and patient report.
Methods
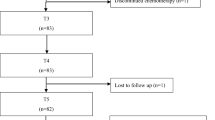
Forty-five taxane-treated patients (F = 43, 66 ± 1.5 years, 19 months post-treatment) completed bilateral neuropathy assessments via clinical examination, sensory nerve conduction studies (NCS), and patient questionnaires. The laterality index (LI) was calculated as a ratio of smaller to larger side-to-side differences.
Results
Neuropathy was reported by 89% of the cohort. On clinical examination, 83% had ≥ 2 abnormalities, with 38–35% having upper or lower limb sensory amplitudes below normative range. Thirty-five percent indicated side-to-side symptom asymmetry; however, there was no significant asymmetry evident on clinical examination (LI Asym = .60 ± .10, Sym = .76 ± .05, NS) and no difference in side-to-side NCS (median LI:Asym = .69 ± .06, Sym = .81 ± .04, NS; Sural LI:Asym = .80 ± .04, Sym = .81 ± .04, NS). Accordingly, there was no statistical association between patient-reported and objective assessment of side-to-side asymmetry, suggesting discordance between patient experience and objective assessment. Similarly, discrepancies in symptom severity between hands and feet were reported by 32% of the cohort. However, patients reporting differences in symptom severity between the hands and feet were just as likely to present with comparable assessments as to demonstrate objective discrepancies.
Conclusions
Discrepancies may exist between the patient experience of CIPN and objective assessments. Understanding these discrepancies may help to elucidate underlying mechanisms and better inform treatment strategies.


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hennenfent KL, Govindan R (2005) Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol 17(5):735–749. https://doi.org/10.1093/annonc/mdj100
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J et al (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63(6):419–437. https://doi.org/10.3322/caac.21204
Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC (1993) Neurotoxicity of Taxol. J Natl Cancer Inst Monogr 15:107–115
Alberti P, Rossi E, Cornblath DR, Merkies ISJ, Postma TJ, Frigeni B, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galiè E, Briani C, Dalla Torre C, Faber CG, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey D, Kerrigan S, Schenone A, Fabbri S, Valsecchi MG, Cavaletti G, CI-PeriNomS Group (2014) Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: two sides of the same coin. Ann Oncol 25(1):257–264. https://doi.org/10.1093/annonc/mdt409
Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lantéri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R, EORTC Quality of Life Group (2005) The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 41(8):1135–1139
Brundage MD, Pater JL, Zee B (1993) Assessing the reliability of two toxicity scales: implications for interpreting toxicity data. J Natl Cancer Inst 85(14):1138–1148
Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM (2004) How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 22(17):3485–3490. https://doi.org/10.1200/jco.2004.03.025
Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7(11):903–909. https://doi.org/10.1016/s1470-2045(06)70910-x
Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D, Crew KD (2011) Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 125(3):767–774. https://doi.org/10.1007/s10549-010-1278-0
Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D (2012) Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Cancer 20(11):2959–2967. https://doi.org/10.1007/s00520-012-1428-5
Smith EML, Knoerl R, Yang JJ, Kanzawa-Lee G, Lee D, Bridges CM (2018) In search of a gold standard patient-reported outcome measure for use in chemotherapy- induced peripheral neuropathy clinical trials. Cancer Control 25(1):1073274818756608. https://doi.org/10.1177/1073274818756608
England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ, American Academy of Neurology, American Association of Electrodiagnostic Medicine, American Academy of Physical Medicine and Rehabilitation (2005) Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 64(2):199–207. https://doi.org/10.1212/01.wnl.0000149522.32823.ea
Cavaletti G, Jann S, Pace A, Plasmati R, Siciliano G, Briani C, Cocito D, Padua L, Ghiglione E, Manicone M, Giussani G, Italian NETox Group (2006) Multi-center assessment of the Total Neuropathy Score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst 11(2):135–141. https://doi.org/10.1111/j.1085-9489.2006.00078.x
Siao P, Cros DMD, Vucic S (2011) Practical approach to electromyography: an illustrated guide for clinicians. vol Book, Whole. Demos Medical, New York
Burke D, Skuse NF, Lethlean AK (1974) Sensory conduction of the sural nerve in polyneuropathy. J Neurol Neurosurg Psychiatry 37(6):647–652
Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, Cella D (2003) Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 13(6):741–748
Webster K, Cella D, Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 1:79. https://doi.org/10.1186/1477-7525-1-79
Bennett B, Goldstein D, Friedlander M, Hickie I, Lloyd A (2007) The experience of cancer-related fatigue and chronic fatigue syndrome: a qualitative and comparative study. J Pain Symptom Manag 34(2):126–135. https://doi.org/10.1016/j.jpainsymman.2006.10.014
Lo YL, Leoh TH, Lim LL, Fook-Chong S, Ng YL, Ratnagopal P (2008) The laterality index in the evaluation of distal sensorimotor neuropathy. J Clin Neuromuscul Dis 10(1):18–21. https://doi.org/10.1097/CND.0b013e3181856306
Argyriou AA, Polychronopoulos P, Iconomou G, Koutras A, Kalofonos HP, Chroni E. Paclitaxel plus carboplatin-induced peripheral neuropathy: a prospective clinical and electrophysiological study in patients suffering from solid malignancies J. Neurol. 2005;252(12):1459. doi:https://doi.org/10.1007/s00415-005-0887-8, 1464
Tzatha E, DeAngelis LM (2016) Chemotherapy-induced peripheral neuropathy. Oncology (Williston Park) 30(3):240–244
Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB (2016) Chemotherapy-induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain. 157(3):560–568. https://doi.org/10.1097/j.pain.0000000000000404
Pachman DR, Qin R, Seisler D, Smith EM, Kaggal S, Novotny P, Ruddy KJ, Lafky JM, Ta LE, Beutler AS, Wagner-Johnston ND, Staff NP, Grothey A, Dougherty PM, Cavaletti G, Loprinzi CL (2016) Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 24(12):5059–5068. https://doi.org/10.1007/s00520-016-3373-1
Wolf SL, Barton DL, Qin R, Wos EJ, Sloan JA, Liu H, Aaronson NK, Satele DV, Mattar BI, Green NB, Loprinzi CL (2012) The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer 20(3):625–632. https://doi.org/10.1007/s00520-011-1141-9
Chen X, Stubblefield MD, Custodio CM, Hudis CA, Seidman AD, DeAngelis LM (2013) Electrophysiological features of Taxane-induced polyneuropathy in patients with breast Cancer. J Clin Neurophysiol 30(2):199–203. https://doi.org/10.1097/WNP.0b013e3182767d3b
Zhi WI, Chen P, Kwon A, Chen C, Harte SE, Piulson L, Li S, Patil S, Mao JJ, Bao T (2019) Chemotherapy-induced peripheral neuropathy (CIPN) in breast cancer survivors: a comparison of patient-reported outcomes and quantitative sensory testing. Breast Cancer Res Treat 178:587–595. https://doi.org/10.1007/s10549-019-05416-4
Goel S, Goldberg GL, Kuo DY, Muggia F, Arezzo J, Mani S (2008) Novel neurosensory testing in cancer patients treated with the epothilone B analog, ixabepilone. Ann Oncol 19(12):2048–2052. https://doi.org/10.1093/annonc/mdn420
Kandula T, Farrar MA, Kiernan MC, Krishnan AV, Goldstein D, Horvath L et al (2017) Neurophysiological and clinical outcomes in chemotherapy-induced neuropathy in cancer. Neurophysiol Clin 128(7):1166–1175. https://doi.org/10.1016/j.clinph.2017.04.009
Park SB, Kwok JB, Asher R, Lee CK, Beale P, Selle F, Friedlander M (2017) Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinician-reported incidence and severity of neurotoxicity in the ICON7 trial. Ann Oncol 28(11):2733–2740. https://doi.org/10.1093/annonc/mdx491
Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, le-Lindqwister N, Gilman PB, Shapiro CL, Alliance for Clinical Trials in Oncology (2013) Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama. 309(13):1359–1367. https://doi.org/10.1001/jama.2013.2813
Kautio AL, Haanpaa M, Leminen A, Kalso E, Kautiainen H, Saarto T (2009) Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res 29(7):2601–2606
Lavoie Smith EM, Haupt R, Kelly JP, Lee D, Kanzawa-Lee G, Knoerl R, Bridges C, Alberti P, Prasertsri N, Donohoe C (2017) The content validity of a chemotherapy-induced peripheral neuropathy patient-reported outcome measure. Oncol Nurs Forum 44(5):580–588. https://doi.org/10.1188/17.Onf.580-588
Hertz DL (2019) Concerns regarding use of patient-reported outcomes in biomarker studies of chemotherapy-induced peripheral neuropathy. Pharm J 19:411–416. https://doi.org/10.1038/s41397-019-0093-1
Lee KM, Jung D, Hwang H, Son KL, Kim TY, Im SA, Lee KH, Hahm BJ (2018) Pre-treatment anxiety is associated with persistent chemotherapy-induced peripheral neuropathy in women treated with neoadjuvant chemotherapy for breast cancer. J Psychosom Res 108:14–19. https://doi.org/10.1016/j.jpsychores.2018.02.012
Velasco R, Petit J, Clapés V, Verdú E, Navarro X, Bruna J (2010) Neurological monitoring reduces the incidence of bortezomib-induced peripheral neuropathy in multiple myeloma patients. J Peripher Nerv Syst 15(1):17–25. https://doi.org/10.1111/j.1529-8027.2010.00248.x
Argyriou AA, Polychronopoulos P, Koutras A, Iconomou G, Iconomou A, Kalofonos HP, Chroni E (2005) Peripheral neuropathy induced by administration of cisplatin- and paclitaxel-based chemotherapy. Could it be predicted? Support Care Cancer 13(8):647–651. https://doi.org/10.1007/s00520-005-0776-9
Park SB, Goldstein D, Lin CS, Krishnan AV, Friedlander ML, Kiernan MC (2009) Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol 27(8):1243–1249. https://doi.org/10.1200/jco.2008.19.3425
Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC (2009) Oxaliplatin-induced neurotoxicity: changes in axonal excitability precede development of neuropathy. Brain. 132(Pt 10):2712–2723. https://doi.org/10.1093/brain/awp219
Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P et al (2014) Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 85(4):392–398. https://doi.org/10.1136/jnnp-2013-305334
Argyriou AA, Park SB, Islam B, Tamburin S, Velasco R, Alberti P et al (2019) Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2019-320969
Funding
This study was supported by a Cancer Institute NSW Program Grant (14/TPG/1-05) and a National Health and Medical Research Council of Australia (NHMRC) Project Grant (#1080521). SBP is supported by a NHMRC Career Development Fellowship (#1148595). MCK was supported by a NHMRC Practitioner Fellowship (1156093); and by ForeFront, a large collaborative research group dedicated to the study of neurodegenerative diseases and funded by the National Health and Medical Research Council of Australia Program Grant (#1132524).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (RPAH Zone of the Sydney Local Health District X14-0239) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Timmins, H.C., Li, T., Kiernan, M.C. et al. Taxane-induced peripheral neuropathy: differences in patient report and objective assessment. Support Care Cancer 28, 4459–4466 (2020). https://doi.org/10.1007/s00520-020-05299-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05299-y