Summary
Isolated pulmonary valve endocarditis (IPE) is a rare form of infectious endocarditis. This article reports the case of a 49-year-old patient with IPE who was initially admitted with suspected cholecystitis. After vegetations were detected by transthoracic (TTE) and transesophageal echocardiography (TEE), antibiotic therapy in accordance with the antibiogram was primarily attempted; however, due to persistently elevated infection parameters and structural valve damage a pulmonary valve replacement was eventually performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isolated pulmonary valve endocarditis (IPE) is a very rare disease with a reported incidence of only 1–2% of all cases of infective endocarditis [1, 2]. Published risk factors include congenital cardiac malformation, right heart failure, sepsis, intravenous drug use, and placement of a central venous catheter or pacemaker [3, 4]. This article describes the case of a 49-year-old IPE patient with systemic bacteremia.
Case description
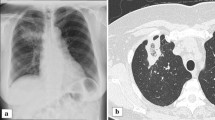
A 49-year-old male patient presented to the emergency department with fever and body aches. Laboratory tests showed increased infection and cholestasis parameters. Sonography revealed cholecystolithiasis, so the patient was admitted to the gastroenterology ward with suspected cholecystitis. An antibiotic regimen was established, but no improvement in the general condition of the patient could be achieved. Computed tomography of the thorax and abdomen revealed pericardial effusion and multiple peripheral pulmonary emboli. Thus, we performed transthoracic (TTE) and transesophageal echocardiography (TEE), where an isolated pulmonary valve endocarditis with a vegetation of 4 cm was detected (Fig. 1; Videos S1–S3). In the microbiological processing of the blood cultures, Streptococcus agalactiae, Staphylococcus haemolyticus and Staphylococcus pettenkoferi were detected. The patient was transferred to the cardiology ward and switched to antibiotic therapy with cefazolin and daptomycin in accordance with the antibiogram. Further diagnostic work-up revealed a carious tooth status and a necrotizing ulcer of the right second toe, most likely due to type 2 diabetes. One tooth was extracted and the ulcerated toe was amputated. Moreover, guideline-recommended antidiabetic therapy was initiated. In consultation with the Department of Cardiothoracic Surgery of our partner hospital, a conservative approach was primarily recommended; however, pulmonary valve replacement with a homograft was eventually performed due to persistently elevated infection parameters and the lack of improvement in echocardiographic findings. In fact, subsequent follow-up TEE, as demonstrated in the attached loops S1–S3, revealed progressive valve destruction with subsequent valve dysfunction.
Vegetation at the pulmonary valve in transthoracic and transesophageal echocardiography. a Parasternal short axis view showing the vegetation on the pulmonary valve (red arrow). b Transesophageal echocardiography showing the vegetation at the pulmonary valve (red arrow) and the tricuspid valve (blue arrow) without any vegetation. c Transesophageal echocardiography showing the mitral valve (green arrow) and tricuspid valve (blue arrow) without any vegetation. d Transesophageal echocardiography showing the aortic valve without any vegetation (purple arrow)
Surgical valve replacement was performed 3 days after the transfer to our partner hospital. A cryopreserved homograft, size 29 mm, (Lifenet Health, Virginia Beach, VA, USA) was implanted. As per standard operating procedure in cases of valve replacement due to endocarditis, cultures were obtained from the valve material; however, microbiological analysis revealed no pathogens on the native valve itself.
Following a 1-day stay in the intensive care unit, the patient was successfully transferred to the cardiothoracic surgery general ward. After a further 8‑day stay, the patient was discharged from inpatient care. The antibiotic therapy, guided by the antibiogram, was administered for 6 weeks.
Comment
IPE is a very rare variant of infective endocarditis, accounting for only 1–2% of all cases of endocarditis [6]. Its low incidence may be due to anatomical or hemodynamic properties of the pulmonary valve. Documented cases indicate that IPE occurs more often in patients with elevated right ventricular pressure, septic conditions, or intravenous drug use, suggesting a predisposing influence [7, 8]. The question why the pulmonary valve was specifically affected in our patient is more complex. In this case, the bacteremia may have been the result of a recent tooth extraction or, more likely, the consequence of a chronic necrotizing ulcer of the right second toe due to type 2 diabetes mellitus. The blood cultures identified Streptococcus agalactiae, Staphylococcus haemolyticus, and Staphylococcus pettenkoferi, all of which are recognized causes of bacterial endocarditis, particularly in the presence of predisposing factors such as dental and skin infections. Yet, the microbiological analysis of the native valve material did not reveal any evidence of pathogens; however, this is frequently seen in patients with endocarditis [5]. A similar case in the literature describes an individual with diabetes mellitus and subsequent sepsis, who was diagnosed with pulmonary valve endocarditis [9] underscoring the significance of systemic infections and pre-existing risk factors in the development of IPE.
The need for surgery in IPE depends on various factors, including severity of the infection, occurrence of complications, response to antibiotic therapy, valve function and the overall clinical status of the patient. In some cases, conservative management with antibiotics alone may be successful, particularly in patients with uncomplicated IPE and a favorable response to medical treatment [6]; however, in cases of significant valve destruction with subsequent valve dysfunction, persistent infection despite appropriate antibiotic therapy, development of complications, such as abscess formation or septic embolization, and failure to achieve clinical improvement, surgical intervention becomes necessary [1, 6, 10]. Empirical evidence has demonstrated that surgical measures, such as valve replacement or repair, are frequently indispensable in cases of IPE to effectively eradicate the infection, mitigate the risk of embolic events, and restore cardiac function [11, 12]. The decision to proceed with surgery is typically made on a case-by-case basis, considering the individual patient’s clinical presentation, imaging findings, and response to medical treatment. In our case, the decision for surgical treatment was made primarily due to the unmanageable sepsis and further valve destruction with subsequent dysfunction in the follow-up TEE. The attending cardiac surgeons opted for a homograft for the following reasons: first, homografts in pulmonary position show excellent hemodynamic results. Second, taking into account the young age of the patient (49 years), a homograft was a good choice in terms of durability. Third, from an infectiology standpoint it was appealing to opt for a homograft in order to reduce the amount of xenogenous material. Finally, a homograft was available in a timely manner.
In conclusion, while conservative management may be successful in some cases of IPE, surgical intervention is often necessary, particularly in cases of severe infection or complications. Further research is needed to refine treatment strategies and improve outcomes for patients with this rare but serious condition.
Data availability statement
No new data were generated or analyzed in support of this research.
References
Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr., Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the international collaboration on endocarditis-prospective cohort study. Arch Intern Med. 2009;169(5):463–73.
Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40(39):3222–32.
Sharma S, Malavia GA. Pulmonary valve infective endocarditis: a case series. Ann Pediatr Cardiol. 2021;14(4):496–500.
Zhang MX, Zhang WM, Yu C, Zhao BW, Chen R, Pan M, et al. Isolated pulmonary valve endocarditis with rapid progression: a case report and literature review. J Cardiothorac Surg. 2021;16(1):16.
Siquier-Padilla J, Cuervo G, Urra X, Quintana E, Hernandez-Meneses M, Sandoval E, et al. Optimal timing for cardiac surgery in infective endocarditis with neurological complications: a narrative review. J Clin Med. 2022;11(18).
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European society of cardiology (ESC). Endorsed by: European association for cardio-thoracic surgery (EACTS), the European association of nuclear medicine (EANM). Eur Heart J. 2015;36(44):3075–128.
Saleem M, Ahmed F, Patel K, Munir MB, Ghaffar YA, Mujahid H, et al. Isolated pulmonic valve endocarditis: case report and review of existing literature on diagnosis and therapy. CASE. 2019;3(5):227–30.
Valente AM, Jain R, Scheurer M, Fowler VG Jr., Corey GR, Bengur AR, et al. Frequency of infective endocarditis among infants and children with staphylococcus aureus bacteremia. Pediatrics. 2005;115(1):e15–9.
Jassal DS, Chiasson M, Rajda M, Ostry A, Legare JF. Isolated pulmonic valve endocarditis. Can J Cardiol. 2005;21(4):365–6.
Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121(9):1141–52.
Revilla A, Lopez J, Sevilla T, Villacorta E, Sarria C, Manzano Mdel C, et al. In-hospital prognosis of prosthetic valve endocarditis after urgent surgery. Rev Esp Cardiol. 2009;62(12):1388–94.
Cahill TJ, Prendergast BD. Haemodialysis is a major risk factor for infective endocarditis—authors’ reply. Lancet. 2016;388(10042):340.
Funding
Open access funding provided by Karl Landsteiner University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Gremmel received speaker fees from Amgen, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Novartis, and Pfizer, and grant support from Boehringer-Ingelheim, Bristol Myers Squibb, Medtronic, and Abbott. S. Valsky, D. Mutschlechner and D. Wiedemann declare that they have no competing interests.
Ethical standards
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated. Written informed consent was obtained from the respective patient.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Video S1: Transthoracic parasternal short axis view showing the vegetation on the pulmonary valve.
Video S2: Transthoracic parasternal short axis view showing the vegetation on the pulmonary valve.
Video S3: Transesophageal echocardiography showing the vegetation on the pulmonary valve and the tricuspid valve without any vegetation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Valsky, S., Mutschlechner, D., Wiedemann, D. et al. Isolated pulmonary valve endocarditis. Wien Klin Wochenschr (2024). https://doi.org/10.1007/s00508-024-02416-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00508-024-02416-3