Summary
Purpose
To establish a transborder virtual tumor board (VTB) fostering state-of-the-art management of cancer patients by exchanging knowledge and expertise among oncologists in Central and Southeastern Europe (CEE).
Methods
We established and implemented a VTB based on the WebEx platform. This allowed for password-protected and secure upload of patient cases to be presented and discussed among colleagues from various oncology centers scattered throughout CEE in order to arrive at a recommendation for further diagnoses and/or treatment.
Results
A total of 73 cases from 16 oncology centers located in 11 CEE countries were uploaded by 22 physicians; 71 were discussed over the course of 17 virtual meetings between June 2018 and May 2019 and 12 different kinds of malignant diseases were discussed with lung cancer (46.6%), melanoma (19.2%) and bladder cancer (13.6%) being the most commonly presented tumor entities. Of the discussed patients, 93.3% had stage IV disease at the time of presentation, 62.6% received chemotherapy or targeted treatment and 67.1% were treated with immune checkpoint inhibitors (ICPIs). The most common causes for presentation and discussion of patient cases were related to the use of ICPIs (80%).
Conclusion
When the need for expertise exceeds locally available resources, web-based VTBs provide a feasible way to discuss patient cases and arrive at conclusions regarding diagnoses and/or treatment across large geographic distances. Moreover, VTBs provide an innovative way for proper, state-of-the-art management of patients with malignant diseases in times of social distancing and the resulting need for restricted interaction during the current SARS-CoV‑2 (severe acute respiratory syndrome coronavirus type 2) pandemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Central European Cooperative Oncology Group (CECOG) has been active in the area of Central and Southeastern Europe (CEE) since 1999. The CECOG has conducted controlled clinical trials, provided continuing medical education and recently also provided analyses regarding access to drugs in an area which is very often characterized by protracted reimbursement decisions on European Medicines Agency (EMA)-registered medicines [1, 2].
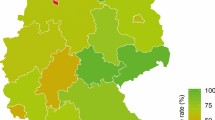
The CEE is a large geographic area (Fig. 1) where access to EMA-registered drugs is hampered by varying national reimbursement strategies. Moreover, quality-oriented day to day decisions in individual cancer care in certain geographic areas may exceed available resources [3].
Participating countries in Central and Southeastern Europe. Shades of blue denote the number of presented cases with darker hues representing more cases (see Table 1); green represents the location of the CECOG Headquarters in Austria. Participating CEE countries: Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Greece, Hungary, Poland, Romania, Serbia, Slovak Republic, Slovenia. CECOG Central European Cooperative Oncology Group, CEE Central and Southeastern Europe
Access to high-quality care in an age of precision medicine and immune checkpoint directed therapeutic interventions with ever growing numbers of indications have become critical in providing state-of-the-art treatment according to not only registrations by the EMA, but also ongoing clinical research. The latter two aspects are of special interest in regions with the constraints of limited resources where decisions on reimbursement are made on national levels. These circumstances pose limitations in clinical experience in drug handling, particularly of recently approved and registered drugs which have added enormously to the treatment armamentarium of malignancies, such as immune checkpoint inhibitors (ICPIs), yet pose a challenge regarding their use, the duration of treatment, decision-making in the case of mixed responses as well as toxicity and its handling.
All the above considerations let to the decision of CECOG to set up a multidiscipilnary, transborder virtual tumor board (VTB) in CEE. This VTB would offer an opportunity to connect oncologists and cancer centers in the form of a virtual network in order to enable appropriate guideline-driven multidisciplinary evaluation and cancer care [4,5,6,7,8] with the main emphasis upon training in the use of ICPIs in order to facilitate access to this important group of newly developed drugs in the region.
The present report describes the methodology used to establish a transborder VTB and its feasibility regarding presented patients, their diagnoses and treatment recommendations in an area of frequently limited financial resources. We believe that such a tool could be used to facilitate and enhance cancer care in other parts of the world with similar or comparable circumstances. Needless to say, the intercurrent SARS-CoV‑2 pandemic would further support the virtual approach.
Methods
Establishment of a transborder VTB
An electronic platform based on the WebEx (Cisco Systems, Inc., San Jose, CA, USA) teleconference system has been established with the help of create-mediadesign GmbH, Vienna, Austria. The platform enabled the password-protected upload of patient histories. The platform was provided with the ability to be joined by clinicians from the area of CEE selected from 150 academic centers in 23 countries partnering with CECOG by virtue of their high expertise in the field. From the very start and according to the topic of the competitively acquired grant, it was made clear that the content of the platform would serve discussions regarding indications and complications of CPI treatment in various malignancies. Initially, a group of 12 selected investigators consisting of chairpersons of oncology departments of high reputation from the area with balanced distribution among various countries were invited to the CECOG headquarters in Vienna, Austria, for setting up the tumor board procedures in February 2018. At this point, the VTB was first presented to a larger audience. These persons were asked to nominate important hospitals with appropriate points of gravitation in the field from each individual country resulting in 22 clinicians from 16 centers in 11 countries in the CEE area. A training session on access to the platform, upload of data and technical aspects of clinicians was subsequently provided to the involved centers.
Implementation of the transborder VTB
At the initial meeting, it was agreed that the large number of participating centers would preclude weekly conferences among all participants. Thus, biweekly meetings with changing participants were agreed. The first tumor board was held in June 2018 and the concept was followed for 1 year during which 73 cases were uploaded to the system and 71 cases were actively discussed; all 73 uploaded cases were evaluated for the purpose of the present report.
Centers and clinicians scheduled for participation were contacted by the CECOG head-office 5 days in advance and asked to upload their cases including patient histories and radiological evaluations.
Each VTB was moderated by the CECOG head office, and a protocol of each tumor board was prepared and uploaded to the platform. Tumor boards were chaired by one of the involved clinicians (C.Z., T.Cu., T.Ci.).
Evaluation of the transborder VTB
Success of the VTB was evaluated using knowledge questionnaires before and after an initial training session on ICPI treatment (spring 2018) and again at the end of the VTB period (spring 2019). A survey was sent to the participants in July 2021 to evaluate the value of the VTB to the participants. The supplement shows all questionnaires and the respective results.
Ethics
All patient data were presented to the VTB anonymized at the level of month/year of birth, ethnicity and biological sex. Patient consent for aggregated evaluation of their cases within the present project was obtained.
Ethics committee approval for conducting these VTB meetings was not required as per local law.
Results
Included cases per country and per site
A total number of 73 cases from 16 oncology centers located in 11 countries in the CEE region were uploaded to the VTB system by 22 physicians and 71 were presented and discussed over the course of 17 virtual meetings between June 11th, 2018 and May 27th, 2019; all 73 uploaded cases are discussed here. On average 4.6 cases from each center and 3.3 cases per physician were presented and discussed (Table 1).
Different types of malignant diseases were recorded (Table 2). Lung cancer (46.6%), melanoma (19.2%) and bladder cancer (13.6%) were the most commonly presented tumor entities and accounted for 80% of all cases. The majority of discussed patient cases had advanced stages of disease, 93.3% had evidence of metastasis at time of presentation, 62.6% had previously received chemotherapy or targeted therapy, 60.3% had undergone surgery, 49.3% had received radiotherapy, 20 patients (27.4%) had undergone all 3 different treatment modalities and 50 patients (67.1%) received ICPI treatment.
Educational value of the VTB
The results of the questionnaires provided before and after the initial training session showed a clear improvement in the percentage of correct responses, with the number of questions answered correctly by fewer than 80% of the respondents decreasing from 12 to 4 (Fig. 2a,b). The follow-up evaluation conducted after approximately 1 year contained a different set of questions with the majority of questions being answered correctly by 80% or more of participants (Fig. 3). The corresponding questions and correct answers can be found in the online supplementary material.
Value of the VTBs to participants
The post-VTB survey was completed by 14 participants from 11 countries. Of 14 respondents, 8 (57%) rated the quality of the VTB meetings as excellent, 5 (36%) as good, 1 (7%) as average. Personal motivations to participate were continuous medical education (n = 6, 43%), specific questions on particular cases (n = 6, 43%), and networking (n = 2, 14%); these motivations were fulfilled in everybody’s view. Especially when their experience with ICPIs was lower, the VTB helped to increase knowledge.
The main motivation to present a particular case was a general clinical case discussion (n = 5, 36%), management of ICPI side effects (n = 4, 29%), and the discussion of ICPI-related treatment options (n = 2, 14%; Fig. 4). Participants’ expectations with regards to their main motivation were met in 86% of cases (n = 12); one respondent each stated no or not applicable. Learning from their own or other participants’ cases related mostly to ICPI side effect management, treatment sequencing and decision making after progression from ICPIs, reasons for deterioration of the disease, and getting confirmation from others of the treatment decision taken.
For future educational sessions, participants suggested to learn on clinical trial initiatives and collaboration with other specialties, treatment sequencing, ICPI side effect management, ICPIs in combination with other treatments, new compounds and other tumor board formats, such as molecular tumor boards.
Discussion
The presented period of TIGER virtual tumor boards was conducted biweekly over 1 year in countries of central and southeastern Europe. At the time participants had little to no personal experience with ICPI therapy. Accordingly, physicians participated for reasons of continuous medical education or because they had specific questions to the VTB, largely around general case discussions and if ICPIs were an option in their presented cases and side effect management. These individual expectations were met in the vast majority of cases.
Tumor boards drawing on the expertise of multidisciplinary teams have become standard worldwide as an approach to discuss complex patient cases and arrive at well-founded treatment decisions in the management of cancer patients. The CECOG was founded in Vienna, Austria, in the year 1999 and established a number of committees with the objective to harmonize among western, central and southeastern European countries the implementation of treatment standards resulting from medical and scientific research in the treatment of cancer patients.
This initiative rests on three pillars: (1) multi-country clinical trials, (2) the CECOG Academy, and (3) a professional society involved in improving cancer care through the support of local reimbursement negotiations, patient advocacy, patient registries, etc. The initial goals of the TIGER VTBs were to improve the understanding of the immune system and its role in cancer. Further we wanted to inform on outcomes of ICPI treatments in various indications and on options of combination therapies to ameliorate treatment outcomes with currently available clinical examples and an outlook on upcoming options, to educate on the use of ICPIs in special clinical situations and to guide participants regarding the management of side effects (https://academy.cecog.org/about-tiger/).
Transborder mentoring initiatives, such as this one, are especially important for clinicians from low to middle-income countries who often do not have experienced colleagues from multiple disciplines in house. Also, they act as networking incubators, especially for the rising generation, and can offer access to clinical trials. An initiative from Latin America followed a similar approach with a web-based pediatric brain tumor board involving clinicians from 15 countries [9]. The most frequently asked questions to the Latin American Tumor Board (LATB) also revolved around questions regarding treatment, second opinions, routine case presentations and alternative diagnostic or therapeutic options. Uncertainty about the best therapeutic approach was the main motivation for the case presentation to experienced colleagues. When treatment options are generally available but difficult to access in a certain region, the personal experience and the respective learning curve are often lagging behind more affluent regions.
Our survey conducted approximately 2 years after the end of the presented period of the VTB clearly shows that this initiative helped when clinicians’ experience was low and increased their knowledge, especially with regard to the management of side effects of the new class of drugs. i.e. ICPIs and the understanding of unexpected disease development.
It is a limitation of the present project that we did not systematically evaluate the recommendations made to the participants, if the VTB confirmed the presented approach or recommended alternative treatments, additional diagnostic testing etc., and if the recommendations were followed and with what outcome. Only few cases (n = 12) were presented a second time for follow-up discussion.
Others have investigated the impact of multidisciplinary tumor boards on the quality of diagnosis and treatment and found that between 4% and 45% of presented cases saw changes in the diagnosis or staging and between 4.5% and 52% recommendations saw changes in the management plan, as summarized by Habermann et al. [10]. The systematic analysis of the feasibility of the recommendations made at the abovementioned LATB showed that only 64% of all recommendations and 60% of diagnostics-related recommendations could be implemented [9], while on-site tumor boards were reported to result in 75–90% of feasible recommendations [11, 12].
Indeed, our survey showed that cost and reimbursement were important obstacles hindering the implementation of the recommendations made. Thus, professional societies and advocacy groups will have to continue in their efforts to forge compromises between payors and the pharmaceutical industry to allow for rapid reimbursement of important new medicines also in low and middle-income countries.
In the current pandemic times, we have learned to embrace virtual means of personal and educational exchange and the available tools have become more user-friendly. Initiatives such as the TIGER VTBs will increase in number and will enhance the interconnectedness of clinicians regionally and worldwide. This knowledge exchange will support clinicians with limited possibilities for personal experience with certain new drugs or diagnostic procedures to swiftly learn from each other and from experienced colleagues. Future VTBs should systematically evaluate the feasibility of recommendations made by the group of experts in order to improve patient outcomes and to identify and facilitate new research initiatives.
Abbreviations
- CECOG:
-
Central European Cooperative Oncology Group
- CEE:
-
Central and Southeastern Europe
- EMA:
-
European Medicines Agency
- ICPIs:
-
Immune checkpoint inhibitors
- NSCLC:
-
Non-small cell lung cancer
- VTB:
-
Virtual tumor board
References
Cufer T, Ciuleanu TE, Berzinec P, et al. Access to novel drugs for non-small cell lung cancer in central and southeastern europe: a central European cooperative oncology group analysis. Oncologist. 2020;25(3):e598–e601.
Wilking N, Bucsics A, Kandolf Sekulovic L, et al. Achieving equal and timely access to innovative anticancer drugs in the European Union (EU): summary of a multidisciplinary CECOG-driven roundtable discussion with a focus on Eastern and South-Eastern EU countries. ESMO Open. 2019;4(6):e550.
Bouvier AM, Bauvin E, Danzon A, et al. Place of multidisciplinary consulting meetings and clinical trials in the management of colorectal cancer in France in 2000. Gastroenterol Clin Biol. 2007;31(3):286–91.
Ricke J, Bartelink H. Telemedicine and its impact on cancer management. Eur J Cancer. 2000;36(7):826–33.
Axford AT, Askill C, Jones AJ. Virtual multidisciplinary teams for cancer care. J Telemed Telecare. 2002;8(Suppl 2):3–4.
Marshall CL, Petersen NJ, Naik AD, et al. Implementation of a regional virtual tumor board: a prospective study evaluating feasibility and provider acceptance. Telemed J E Health. 2014;20(8):705–11.
Stevenson MM, Irwin T, Lowry T, et al. Development of a virtual multidisciplinary lung cancer tumor board in a community setting. J Oncol Pract. 2013;9(3):e77–e80.
Salami AC, Barden GM, Castillo DL, et al. Establishment of a regional virtual tumor board program to improve the process of care for patients with hepatocellular carcinoma. J Oncol Pract. 2015;11(1):e66–e74.
Rosabal-Obando M, Osorio DS, Lassaletta A, et al. Follow-up evaluation of a web-based pediatric brain tumor board in Latin America. Pediatr Blood Cancer. 2021;68(9):e29073.
Habermann TM, Khurana A, Lentz R, et al. Analysis and impact of a multidisciplinary lymphoma virtual tumor board. Leuk Lymphoma. 2020;61(14):3351–9.
Hollunder S, Herrlinger U, Zipfel M, et al. Cross-sectional increase of adherence to multidisciplinary tumor board decisions. Bmc Cancer. 2018;18(1):936.
Chan MH, Boop F, Qaddoumi I. Challenges and opportunities to advance pediatric neuro-oncology care in the developing world. Childs Nerv Syst. 2015;31(8):1227–37.
Acknowledgements
Ursula Fischer of CECOG was involved in the planning and management of the virtual tumor board meetings. Fabian Fischer prepared and analyzed the surveys and was funded by CECOG. Margit Hemetsberger, hemetsberger medical services, Vienna, Austria, provided editorial and writing assistance, funded by CECOG.
Funding
This CECOG initiative was made possible by a competitive educational grant from Pfizer Inc., Grant ID 34580627.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
C. Thallinger and C. Zielinski prepared the draft. All authors were involved in and contributed to the drafting and critical review of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A. Dan, E. Bicakcic, G. Fabian, Z. Kahan, G. Penthedourakis, J. Sgouros, C. Thallinger and A. Wrona declare that they have no competing interests. L.N. Gales declares lecture fees from Actavis, Angelini, Amgen, AstraZeneca, Astellas, BMS, Boehringer, Bayer, Ipsen, J&J, Magnapharm, Merck, MSD, Novartis, Pfizer, Roche, Sanofi, Sandoz, and Servier. M. Mencinger declares honoraria/lecture fees from BMS, Janssen, Astellas, Roche, Pfizer, Sanofi, Amgen, Bausch Health, and Novartis. C.G. Kuhar declares honoraria/lecture fees from Roche, Pfizer, Novartis, Lilly, MSD, LEK, KRKA, Merck, Bayer, and BMS. D. Sirbu declares honoraria/lecture fees from BMS, Novartis, Pfizer, Roche, Ipsen, AstraZeneca, Sandoz, Lilly, Astellas, and Merck. U. Janzic declares honoraria/lecture fees from MSD, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, and Takeda. P. Berzinec declares grants from Roche; honoraria/fees from Amgen, Bayer, Roche, and Takeda; participation in a company sponsored speaker’s bureau from AstraZeneca, Boehringer Ingelheim, and MSD. M. Vosmik declares grants from BMS; honoraria/fees from BMS, MSD, Sanofi, Eli Lilly; participation in a company sponsored speaker’s bureau from BMS, MSD, and Sanofi. C. Zielinski declares honoraria/fees from Athenex, MSD, Imugene, AstraZeneca, Servier, and Eli Lilly. L. Simetic declares grants from MSD, Novartis, BMS, Genesis Pharma, and Roche; honoraria/fees from MSD, Novartis, BMS, Genesis Pharma, and Roche.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability statement
Qualified researchers may request data from research conducted under the auspices of the CECOG by contacting the corresponding author.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thallinger, C., Berzinec, P., Bicakcic, E. et al. Establishment of a virtual transborder tumor board for cancer patients in Central and Southeastern Europe. Wien Klin Wochenschr 134, 697–704 (2022). https://doi.org/10.1007/s00508-022-02016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-022-02016-z