Summary
Background
Acute viral myositis (AVM) may be triggered by influenza A/B, enteroviruses and other viruses. Severe complications including rhabdomyolysis regularly lead to acute kidney injury (AKI). The aim of this short report was to discuss management and differential diagnosis of massive creatine kinase (CK) elevation.
Patient, material and methods
Herein, we report on a 19-year-old Austrian male of African descent with a history of respiratory tract infections and whole-body pain. He further developed acute viral myositis and massive CK elevation up to 440,000 IU/L but without any signs of AKI. A literature search relating AVM, management and differential diagnosis of rhabdomyolysis was conducted in PubMed and UptoDate.
Results
A full panel of serological and autoimmune blood work-up including testing for human immunodeficiency virus (HIV), hepatitis, influenza A/B, Epstein-Barr virus (EBV), antinuclear antibodies (ANA) and autoantibodies against various extractable nuclear antigens (ENA) did not reveal evidence for viral originators or autoimmune diseases. This case indicates that in acute viral myositis associated with extreme CK elevation (>400,000 IU/L) AKI might be completely absent. Potential causes for this clinical phenotype, differential diagnosis and management are discussed.
Similar content being viewed by others
Introduction
Acute viral myositis may be complicated by rhabdomyolysis. Several reports demonstrated viral infections caused by influenza A/B, enteroviruses, Epstein-Barr virus and other viruses [1]. The coronavirus SARS-CoV‑2 may also induce neuromuscular symptoms including myalgia and myositis [2]. While the detailed molecular mechanisms leading to myositis remain to be resolved, direct viral-mediated muscle cytotoxicity and/or elevation of myotoxic cytokines might be involved [3]. The AVM displays a wide range of clinical features: in severe cases rhabdomyolysis and myoglobinuric acute kidney injury may occur [4]. Typically, patients present with a history of a viral infection, weakness and myalgia most commonly affecting the back and legs along with pigmenturia. Furthermore, creatine kinase levels may rise up to 500,000 IU/L in extreme cases [3]. Here, we discuss the management and differential diagnosis of massive CK elevation in an affected patient. We report AVM after anamnestic respiratory tract and EBV infections with severe CK elevation (>400,000 IU/L) in the presence of stable kidney function.
Patient, material and methods
The patient (19 years old, African descent, male) was admitted to the Clinic Ottakring, Vienna, Austria in January 2020 due to the suspicion of urinary tract infection (nitrite positivity of a spot urine specimen), pigmenturia and severe diffuse muscle pain. He displayed a likely history of a recent viral respiratory tract infection, doubtful chlamydia pneumonia, with positive IgA and IgG antibody titers and EBV infection 5 months ago (EBV-IgG positive) with hepatosplenomegaly diagnosed by ultrasound, elevated liver enzymes (AST, ALT, γ‑GT) and general malaise diagnosed by the general practitioner. While devoid of any chronic diseases and any long-term medication, the patient denied excessive amounts of sport, consumption of anabolic agents or other drugs. Also, trauma and history of epileptic seizure were excluded. There was no family history of rheumatic diseases and kidney parameters were normal at the emergency department. Physical examination (vital parameters, temperature, cardiopulmonary and musculoskeletal examination), detailed blood work-up including hemogram, blood sugar, lipid profile and viral serology was performed. Among antiviral antibodies the following were assessed: HIV, hepatitis A/B/C, influenza A/B, EBV, VZV, HSV and parvovirus. Furthermore, autoimmune antibodies were determined including antinuclear antibodies (ANA, ENA, Mi‑2 antibodies and Jo‑1 antibodies). Finally, CK, LDH, liver (AST, ALT, γ‑GT, bilirubin, alkaline phosphatase) and kidney function parameters (serum creatinine, blood urea nitrogen and eGFR MDRD formula), microscopic urine analysis and urine culture were completed. Chest X‑ray and kidney ultrasound were performed. For the literature search involving AVM, management and differential diagnosis, we searched PubMed and UptoDate.
Results
Physical examination and diagnostic assessment
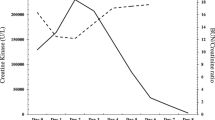
At presentation, the patient was afebrile and vital parameters were unremarkable except for sinus tachycardia (115/min). Cardiopulmonary and musculoskeletal examinations were unremarkable. The urine was macroscopically brown without dysuria. Pain was aggravated during the night and in the morning the patient felt joint stiffness over 1h. Blood work revealed profound elevation of CK with 440,000 IU/L; LDH, AST, ALT, γ‑GT and CRP were also profoundly elevated (Table 1). Serological testing showed positive EBV, VZV, HSV and parvovirus IgG antibodies, IgM antibodies and autoimmune markers were negative. Chest X‑ray and kidney ultrasound were unremarkable. Initial urine dipstick displayed erythrocytes, urobilinogen, nitrite and proteinuria while microscopic urine analysis revealed only a modest elevation of erythrocytes. Urine culture did not show any proof of germs, further smears and blood work excluded Chlamydia trachomatis and Neisseria gonorrhoeae.
Interventions and follow-up
Rhabdomyolysis (CK elevation, pigmenturia and muscle pain) was diagnosed and treated with potassium sodium hydrogen citrate and increased fluid intake up to 3L per day. Furthermore, 400 mg dexibuprofen was administered twice a day (1000 mg metamizole on request up to three times a day) for pain management. CK, LDH, CRP and urine parameters (erythrocytes, total protein, urobilinogen, albumin-creatinine ratio) decreased steadily. Levels of AST and γ‑GT were decreasing but AST, γ‑GT and ALT levels were still elevated at the time of discharge. Remarkably, kidney parameters remained stable throughout the whole inpatient stay and no signs of rhabdomyolysis-induced myoglobinuric AKI were detected. After continuous improvement in both laboratory parameters (CK, LDH, CRP, AST) and complete resolution of muscle pain, the patient was discharged after 13 days. The patient was contacted for a follow-up (November 2020) and serum CK was still slightly elevated while liver transaminases, kidney parameters and albumin-to-creatinine ratio were unremarkable. The patient was in good general condition and did not report further episodes of myalgia or pigmenturia.
Discussion
An AVM is most commonly associated with influenza A/B and enteroviruses but can also be caused by other viruses including EBV, parainfluenza and HSV [1]. Clinical diagnosis of AVM is challenging due to a lack of serological confirmation of viral originators, extremely variable differential diagnosis of rhabdomyolysis origin and absence of specific diagnostic findings for virus-induced myositis in muscle biopsy. Affected patients might first clinically present with constitutional symptoms (fatigue, fever, malaise) due to virally induced systemic cytokines [3, 5]. Rhabdomyolysis may result from traumatic or nontraumatic causes and involves the breakdown of muscle tissue causing intracellular protein leakage; however, the classical triad of symptoms, weakness, brown colored urine and muscle pain, does not regularly occur. Although many differential diagnoses of rhabdomyolysis exist, the most common etiologies in adults are excessive exercise, trauma, seizures, alcohol, drug abuse and infections. Rhabdomyolysis is often complicated by AKI and subsequent electrolyte abnormalities, arrhythmia and compartment syndrome [5]. Depending on the cause and clinical setting 13–50% of traumatic and nontraumatic rhabdomyolysis lead to AKI that significantly increases the mortality risk [6, 7]. Myoglobin released into the bloodstream due to damage of myocytes is central for the pathogenesis of AKI. Excessive free myoglobin is freely filtered exerting direct tubulotoxic effects including tubular obstruction after interacting with uromodulin and intrarenal vasoconstriction by scavenging nitrous oxide [7, 8]. Fodili et al. reported that about 30% of cases with coxsackie virus infection develop AKI as a complication of rhabdomyolysis [9]. The AKI prevalence due to rhabdomyolysis associated with influenza infection is even higher with up to 73% of reported cases developing AKI [10]. A large meta-analysis demonstrated a significant correlation between serum CK levels and the likelihood of rhabdomyolysis-induced AKI especially in traumatic cases [11]. When coexisting conditions, such as sepsis, dehydration, and acidosis are present, even a CK level as low as 5000 U/l may lead to AKI [8]. We report a 19-year-old male with diffuse body pain, a history of both EBV and respiratory tract infection and severe CK elevation but without any signs of AKI despite the classical triad of rhabdomyolysis with weakness, myalgia and pigmenturia. Extrinsic and intrinsic causes for rhabdomyolysis were clinically or serologically excluded, autoimmune markers were unremarkable and the clinical presentation with a history of respiratory infection were in line with the diagnosis of AVM with subsequent rhabdomyolysis. Finally, muscle biopsy is dispensable to diagnose AVM, since no pathognomonic findings for virus-induced myositis exist [1, 3]. Fortunately, viral myositis progressing to rhabdomyolysis is rare [3]. Rhabdomyolysis needs to be considered in viral myositis to start early preventive and supportive treatment. Treatment approaches include aggressive intravenous fluids, urine alkalization and possibly forced diuresis with loop diuretics, while renal replacement therapy is necessary in oligoanuric AKI [5]. Our patient, even with the use of nonsteroidal anti-inflammatory drugs (NSAID), did not develop AKI. Early administration of IVF and alkalization might have helped to prevent development of AKI. Transient proteinuria as observed in the urine dipstick may be interpreted as overflow myoglobin production exceeding tubular resorptive capacity [12]. Transiently elevated ACR indicates increased glomerular capillary permeability and/or diminished proximal tubular reabsorption, but its utility as a test for the early diagnosis of AKI has not been evaluated [13, 14]. Distinct and sensitive biomarkers predictive for AKI development including NGAL and KIM‑1, however, were not studied potentially indicating discrete forms of early AKI. Future studies should therefore examine the role of newer AKI biomarkers in rhabdomyolysis-mediated AKI [15]. Importantly, ethnic differences with respect to CK reference concentrations must also be considered. Hence, CK levels may be up to twice as high in the black population, especially in black men. George et al. report the odds ratio for black individuals for increased CK was higher than in the white study population possibly due to altered clearance and/or production of CK due to a higher tissue CK activity. In addition, the study supports evidence that different ethnic CK levels may not be caused by differences in body compositions [16]. Regarding the literature, we conclude that this case belongs to the spectrum of cases with highly increased clinical severity without myoglobinuric AKI even in the presence of NSAIDs. In patients with a history of viral infections especially of the upper respiratory tract and nonspecific general symptoms, such as weakness and fever, it is vital to assess CK levels once muscle weakness or body pain appear; however, even extreme increases of CK may not predict progression to AKI at least with routine clinical and serological parameters.
Abbreviations
- ACR:
-
Albumin-to-creatinine ratio
- AKI:
-
Acute kidney injury
- ALT:
-
Alanine aminotransferase
- ANA:
-
Antinuclear antibodies
- AST:
-
Aspartate aminotransferase
- AVM:
-
Acute viral myositis
- CK:
-
Creatine kinase
- CRP:
-
C‑reactive protein
- EBV:
-
Epstein-Barr virus
- eGFR:
-
Estimated glomerular filtration rate
- ENA:
-
Autoantibodies against various extractable nuclear antigens
- HIV:
-
Human immunodeficiency virus
- HSV:
-
Herpes simplex virus
- IgA:
-
Immunoglobulin A
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IU/L:
-
International units per liter
- IVF:
-
Intravenous fluid
- γ‑GT:
-
Gamma glutamyl transferase
- KIM‑1:
-
Kidney injury molecule-1
- LDH:
-
Lactate dehydrogenase
- MDRD:
-
Modification of diet in renal disease
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- PCR:
-
Polymerase chain reaction
- SARS-CoV‑2:
-
Severe acute respiratory syndrome coronavirus‑2
- STD:
-
Sexually transmitted diseases
- VSV:
-
Varicella zoster virus
References
Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473–94.
Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol Sci. 2020;41(11):3039–56.
UpToDate, editor. Overview of viral myositis. https://www.uptodate.com/contents/overview-of-viral-myositis?search=acute%20viral%20myositit&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2. Accessed 22 Feb 2020.
Nelson TV, Blaceri S, Biederman JI. Rhabdomyolysis and acute renal failure with syphilitic myositis. Kidney Int. 2016;89(5):1169.
Nauss MD, Schmidt EL, Pancioli AM. Viral myositis leading to rhabdomyolysis: a case report and literature review. Am J Emerg Med. 2009;27(3):372.e5–372.e6.
Cervellin G, Comelli I, Benatti M, Sanchis-Gomar F, Bassi A, Lippi G. Non-traumatic rhabdomyolysis: background, laboratory features, and acute clinical management. Clin Biochem. 2017;50(12):656–62. Aug.
Rodríguez E, Soler MJ, Rap O, Barrios C, Orfila MA, Pascual J. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One. 2013;8(12):e82992.
Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361(1):62–72.
Fodili F, van Bommel EFH. Severe rhabdomyolysis and acute renal failure following recent Coxsackie B virus infection. Neth J Med. 2003;61(5):177–9.
Pesik NT, Otten EJ. Severe rhabdomyolysis following a viral illness: A case report and review of the literature. J Emerg Med. 1996;14(4):425–8.
Safari S, Yousefifard M, Hashemi B, Baratloo A, Forouzanfar MM, Rahmati F, et al. The value of serum creatine kinase in predicting the risk of rhabdomyolysis-induced acute kidney injury: a systematic review and meta-analysis. Clin Exp Nephrol. 2016;20(2):153–61.
Parikh CR, Lu JC, Coca SG, Devarajan P. Tubular proteinuria in acute kidney injury: a critical evaluation of current status and future promise. 2010. https://journals-sagepub-com.ez.srv.meduniwien.ac.at/doi/10.1258/acb.2010.010076?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed. Accessed 23 Nov 2020.
Toto RD. Microalbuminuria: definition, detection, and clinical significance. J Clin Hypertens. 2004;6(11 Suppl 3):2–7.
Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and Albuminuria: really! J Am Soc Nephrol. 2014;25(3):443–53.
Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clin Chim Acta. 2015;438:350–7.
George MD, McGill N‑K, Baker JF. Creatine kinase in the U.S. population: Impact of demographics, comorbidities, and body composition on the normal range. Medicine. 2016;95(33):e4344.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Kietaibl, M. Fangmeyer-Binder, G. Göndör, M. Säemann and P. Fasching declare that they have no competing interests.
Ethical standards
Written informed consent was obtained from the patient for publication of this case report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kietaibl, AT., Fangmeyer-Binder, M., Göndör, G. et al. Acute viral myositis: profound rhabdomyolysis without acute kidney injury. Wien Klin Wochenschr 133, 847–850 (2021). https://doi.org/10.1007/s00508-021-01866-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-021-01866-3