Summary
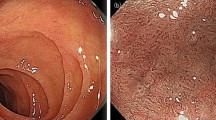
Over the past 5 years several case reports and cohort studies have been published that describe a sprue-like enteropathy (SLE) with abdominal pain, chronic diarrhea and weight loss, after taking angiotensin type 1 receptor blockers (ARB). The initial case series from the Mayo Clinic, which described 22 cases of olmesartan-induced SLE, was followed by numerous case descriptions for almost all ARBs. A total of 73 case reports have been described so far that show evidence of ARB-associated enteropathy with villous atrophy and full recovery 3–12 months after discontinuation of the sartan in question. Of these, 59 cases were olmesartan-associated cases of SLE, another 14 case reports have been published for telmisartan (4), valsartan (3), losartan (2), candesartan (1) and eprosartan (1). Based on the available cohort studies, ARB-associated intestinal malabsorption occurs in 9.8–14 cases per 100,000 patients treated with ARBs, the number treated to harm is over 31,000 patient-years. Although the majority of case reports have been published with olmesartan, in the cohort studies no clear difference within the ARBs can be ruled out. The SLE is rare but must be suspected and diagnostically investigated in every patient presenting with abdominal discomfort, chronic diarrhea and weight loss who is being treated with an ARB, after other causes have been ruled out. In such cases, discontinuing the ARB leads to a full recovery.

Similar content being viewed by others
Abbreviations
- α1:
-
Alpha-1-adrenoceptor
- ARB:
-
Angiotensin receptor‑1 antagonist
- AT1:
-
Angiotension-receptor‑1
- AT2:
-
Angiotension-receptor‑2
- CL−:
-
Chloride
- ECV:
-
Extracellular fluid volume
- K+:
-
Potassium
- NA+:
-
Sodium
- PG:
-
Prostaglandins
- SLE:
-
Sprue-like enteropathy
- TGF beta:
-
Transforming growth factor beta
- TNF-alpha:
-
Tumor necrosis factor alpha
References
Freeman HJ. Drug-induced Sprue-like intestinal disease. Int J Celiac Dis. 2014;2:49–53.
Adike A, Corral J, Rybnicek D, Sussman D, Shah S, Quigley E. Olmesartan-induced Enteropathy. Methodist Debakey Cardiovasc J. 2016;12:230–2.
Scialom S, Malamut G, Meresse B, et al. Gastrointestinal disorder associated with Olmesartan mimics Autoimmune Enteropathy. PLoS ONE. 2015;10:e125024.
Ianiro G, Bibbo S, Montalto M, Ricci R, Gasbarrini A, Cammarota G. Systematic review: Sprue-like enteropathy associated with olmesartan. Aliment Pharmacol Ther. 2014;40:16–23.
Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732–8.
Fiorucci G, Puxeddu E, Colella R, Reboldi PG, Villanacci V, Bassotti G. Severe spruelike enteropathy due to olmesartan. Rev Esp Enferm Dig. 2014;106:142–4.
DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108:647–53.
Dreifuss SE, Tomizawa Y, Farber NJ, Davison JM, Sohnen AE. Spruelike enteropathy associated with olmesartan: an unusual case of severe diarrhea. Case Rep Gastrointest Med. 2013;2013:1–3.
Fabian E, Schiller D, Wenzl H, et al. Clinical-Pathological Conference Series from the Medical University of Graz: Case No 156: 82-year-old woman with chronic diarrhea and weight loss of 20 kilograms. Wien Klin Wochenschr. 2015;127:974–80.
Basson M, Mezzarobba M, Weill A, et al. Severe intestinal malabsorption associated with olmesartan: a French nationwide observational cohort study. Gut. 2016;65:1664–9.
Marthey L, Cadiot G, Seksik P, et al. Olmesartan-associated enteropathy: results of a national survey. Aliment Pharmacol Ther. 2014;40:1103–9.
Mondet L, Pierson-Marchandise M, Gras V, et al. Angiotensin II receptor blockers-induced enteropathy: not just olmesartan! A case report with candesartan. Fundam Clin Pharmacol. 2016;30:62.
Maier H, Hehemann K, Vieth M. Celiac disease-like enteropathy due to antihypertensive therapy with the angiotensin-II receptor type 1 inhibitor eprosartan. Cesk Patol. 2015;51:87–8.
Cammarota G, Ianiro G, Bibbo S, Letter GA. telmisartan associated enteropathy—is there any class effect? Authors’ reply. Aliment Pharmacol Ther. 2014;40:570.
Negro A, Rossi GM, Santi R, Iori V, De Marco L. A case of severe Sprue-like Enteropathy associated with Losartan. J Clin Gastroenterol. 2015;49:794.
Herman ML, Rubio-Tapia A, Wu TT, Murray JA. A case of severe Sprue-like Enteropathy associated with Valsartan. Acg Case Rep J. 2015;2:92–4.
Sawant AP, Spoletini G, Etherington C. Losartan-associated spruelike enteropathy in a post-lung transplant cystic fibrosis patient. J Cyst Fibros. 2017;16:135.
Cyrany J, Vasatko T, Machac J, Nova M, Szanyi J, Kopacova M. Letter: telmisartan-associated enteropathy—is there any class effect? Aliment Pharmacol Ther. 2014;40:569–70.
Mandavdhare HS, Sharma V, Prasad KK, Kumar A, Rathi M, Rana SS. Telmisartan-induced sprue-like enteropathy: a case report and a review of patients using non-olmesartan angiotensin receptor blockers. Intest Res. 2017;15:419–21.
De Bortoli N, Ripellino C, Cataldo N, Marchi S. Unspecified intestinal malabsorption in patients treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers: a retrospective analysis in primary care settings. Expert Opin Drug Saf. 2017;16:1221–5. https://doi.org/10.1080/14740338.2017.1376647.
Malfertheiner P, Ripellino C, Cataldo N. Severe intestinal malabsorption associated with ACE inhibitor or angiotensin receptor blocker treatment. An observational cohort study in Germany and Italy. Pharmacoepidemiol Drug Saf. 2018;27(6):581–586. https://doi.org/10.1002/pds.4402
You SC, Park H, Yoon D, Park S, Joung B, Park RW. Olmesartan is not associated with the risk of enteropathy: a Korean nationwide observational cohort study. Korean J Intern Med. 2019;34(1):90-98. https://doi.org/10.3904/kjim.2017.002
Levens NR. Control of intestinal absorption by the renin-angiotensin system. Am J Physiol. 1985;249:G3–15.
Fandriks L. The renin-angiotensin system and the gastrointestinal mucosa. Acta Physiol (oxf). 2011;201:157–67.
Fandriks L. The angiotensin II type 2 receptor and the gastrointestinal tract. J Renin Angiotensin Aldosterone Syst. 2010;11:43–8.
Hallersund P, Elfvin A, Helander HF, Fandriks L. The expression of renin-angiotensin system components in the human gastric mucosa. J Renin Angiotensin Aldosterone Syst. 2011;12:54–64.
Hallersund P, Helander HF, Casselbrant A, Edebo A, Fandriks L, Elfvin A. Angiotensin II receptor expression and relation to Helicobacter pylori-infection in the stomach of the Mongolian gerbil. Bmc Gastroenterol. 2010;10:3.
Marietta EV, Nadeau AM, Cartee AK, et al. Immunopathogenesis of olmesartan-associated enteropathy. Aliment Pharmacol Ther. 2015;42:1303–14.
Esteve M, Temino R, Carrasco A, et al. Potential coeliac disease markers and autoimmunity in olmesartan induced enteropathy: a population-based study. Dig Liver Dis. 2016;48:154–61.
Ebrahim VS, Martin J, Murthy S, Odstrcil E, Huang H, Polter D. Olmesartan-associated enteropathy. Proc (bayl Univ Med Cent). 2017;30:348–50.
Burbure N, Lebwohl B, Arguelles-Grande C, Green PH, Bhagat G, Lagana S. Olmesartan-associated sprue-like enteropathy: a systematic review with emphasis on histopathology. Hum Pathol. 2016;50:127–34.
Sun L, Wang W, Xiao W, Liang H, Yang Y, Yang H. Angiotensin II induces apoptosis in intestinal epithelial cells through the AT2 receptor, GATA‑6 and the Bax pathway. Biochem Biophys Res Commun. 2012;424:663–8.
Palmquist S, Mathews B. Isolated intestinal type angioedema due to ACE-inhibitor therapy. Clin Case Rep. 2017;5:707–10.
Locascio EJ, Mahler SA, Arnold TC. Intestinal angioedema misdiagnosed as recurrent episodes of gastroenteritis. West J Emerg Med. 2010;11:391–4.
Talbot GH. Small bowel histopathologic findings suggestive of celiac disease in an asymptomatic patient receiving olmesartan. Mayo Clin Proc. 2012;87:1231–2. author reply 2.
Cartee AK, Genere RJP, Oxentenko AS. New onset villous atrophy in a patient with celiac disease. Baillieres Clin Gastroenterol. 2018;155:988–9.
Menne J, Haller H. Olmesartan and intestinal adverse effects in the ROADMAP study. Mayo Clin Proc. 2012;87:1230–1. author reply 2.
Theophile H, David XR, Miremont-Salame G, Haramburu F. Five cases of sprue-like enteropathy in patients treated by olmesartan. Dig Liver Dis. 2014;46:465–9.
Eusebio M, Caldeira P, Antunes AG, et al. Olmesartan-induced Enteropathy: an unusual cause of villous atrophy. Ge Portuguese J Gastroenterol. 2016;23:91–5.
Basson M, et al. Gut. 2015;0:1–6.
Ripellino C, Cataldo N, Scarpignato C. Unspecified intestinal malabsortion associated with angiotensin receptor blocker therapy (Abstract). Quintiles IMS, 2016, https://www.rwebibliography.com/print.php?&totalRows_Bibliographies=101&conditionid=7
Marild K, Lebwohl B, Green PH, Murray JA, Ludvigsson JF. Blockers of Angiotensin other than Olmesartan in patients with villous atrophy: a nationwide case-control study. Mayo Clin Proc. 2015;90:730–7.
Mondet L, Pierson-Marchandise, Gras V, Sevester H, Lawson P, Andrejak M, Masmoudi K. Angiotensin II receptor blcokers-induced enteropathy: not just olmesartan! A case report with candesartan. Fund Clin Pharmacol. 2016;30:67
Famularo G, Minisola G. Relapsing Olmesartan-Associated Ileitis. Ann Pharmacother. 2016;50:1070.
van Gils T, Robijn RJ, Bouma G, Neefjes-Borst EA, Mulder CJJ. A pitfall in suspected (refractory) celiac disease: Losartan-induced Enteropathy. Am J Gastroenterol. 2017;112:1754–5.
Negro A, De Marco L, Cesario V, Santi R, Boni MC, Zanelli M. A case of moderate Sprue-like Enteropathy associated with Telmisartan. J Clin Med Res. 2017;9:1022–5.
Zanelli M, Negro A, Santi R, et al. Letter: sprue-like enteropathy associated with angiotensin II receptor blockers other than olmesartan. Aliment Pharmacol Ther. 2017;46:471–3.
Dong YH, Jin Y, Tsacogianis TN, He M, Hsieh PH, Gagne JJ. Use of olmesartan and enteropathy outcomes: a multi-database study. Aliment Pharmacol Ther. 2018;47:792–800.
Greywoode R, Braunstein ED, Arguelles-Grande C, Green PH, Lebwohl B. Olmesartan, other antihypertensives, and chronic diarrhea among patients undergoing endoscopic procedures: a case-control study. Mayo Clin Proc. 2014;89:1239–43.
Kamal A, Fain C, Park A, et al. Angiotensin II receptor blockers and gastrointestinal adverse events of resembling sprue-like enteropathy: a systematic review. Gastroenterol Rep (oxf). 2019;7:162–7.
Mondet I, Pierson-Marchandise M, Gras V, H. S, Lawsan P. Angiotensin II receptor blockers-induced enteropathy: not just olmesartan! Fundam Clin Pharmacol. 2016;30:47–62.
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72.
Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–78.
Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–6.
Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):1.
Cummins AG, Roberts-Thomson IC. Prevalence of celiac disease in the Asia-Pacific region. J Gastroenterol Hepatol. 2009;24:1347–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.R. Wenzel: For the following companies there is a conflict of interest with the presented data due to consultancy fees or sponsoring: Actelion, Astra-Zeneca, Abbott, Bayer, Daichii-Sankyo, Gepamed, Medtronic, Menarini, Novartis, Speedel Pharma, St Jude Medical and Takeda. C. Datz declares that he has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wenzel, R.R., Datz, C. Association of sprue-like enteropathy and angiotensin receptor-1 antagonists. Wien Klin Wochenschr 131, 493–501 (2019). https://doi.org/10.1007/s00508-019-01539-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-019-01539-2