Abstract
PFAS are a class of synthetic chemicals used for many industrial and domestic purposes. However, once released in the environment, they are persistent, mobile and toxic. One of the most important transport routes to drinking water is through riverbank filtration. Although this is usually an effective strategy for removing many organic compounds, its effectiveness in removing PFAS is still unknown. The aim of this study is to investigate the occurrence, as well as the spatial distribution of PFAS at riverbank filtration sites and compare these to two pharmaceuticals and various chemical parameters. A one-year sampling campaign was carried out at four transects with different characteristics along the Danube river. Samples were analysed using Liquid Chromatography Mass Spectrometry.
Results show that most of the detected PFAS compounds had concentrations lower than 10 ng/l. NaADONA had the highest concentration at all the sites, indicating the presence of an emission source upstream of the monitored sites. For most compounds, there was no concentration reduction between the river and groundwater, implying that no removal processes take place. This was further confirmed with statistical tests, which showed no significant differences between river- and groundwater concentrations. Two sites in Budapest showed higher concentrations of PFOA, PFOS, and GenX in the background water compared to the river, indicating an inland source of these compounds. The current situation imposes no problems for drinking water as the measured concentrations are lower than the legal limit set by the EU Drinking Water Directive. However, any future legal or industrial changes could create problems since results suggest that these compounds are not removed during riverbank filtration.
Zusammenfassung
PFAS sind eine Klasse von synthetischen Chemikalien, die für viele industrielle und häusliche Zwecke verwendet werden. In der Umwelt sind sie persistent, mobil und toxisch. Einer der wichtigsten Transportwege ins Trinkwasser ist die Uferfiltration an Flüssen. Obwohl diese normalerweise eine wirksame Strategie zur Entfernung vieler organischer Verbindungen ist, ist ihre Wirksamkeit bei der Entfernung von PFAS noch unbekannt. Ziel dieser Studie ist es, das Vorkommen und die räumliche Verteilung von PFAS an Standorten mit Uferfiltration zu untersuchen und mit zwei Pharmazeutika und verschiedenen chemischen Parametern zu vergleichen. Eine einjährige Probenahmekampagne wurde an vier unterschiedlichen Transekten mit Uferfiltration entlang der Donau durchgeführt. Die Proben wurden mittels Flüssigchromatographie-Massenspektrometrie analysiert.
Die Ergebnisse zeigen, dass die meisten der nachgewiesenen PFAS-Verbindungen Konzentrationen von weniger als 10 ng/l aufwiesen. NaADONA wies an allen Standorten die höchste Konzentration auf, was auf das Vorhandensein einer Emissionsquelle stromaufwärts der überwachten Standorte hinweist. Bei den meisten Verbindungen gab es keine wesentliche Reduktion der Konzentrationen auf der Fließstrecke vom Fluss ins Grundwasser. Dies zeigt die geringe Bedeutung von Entfernungsprozessen im Bereich der Uferfiltration für PFAS, welche auch durch statistische Tests bestätigt werden konnte, die keine signifikanten Unterschiede zwischen Fluss- und Grundwasserkonzentrationen ergaben. An zwei Standorten in Budapest wurden im Vergleich zum Fluss und den direkt durch Uferfiltration beeinflussten Monitoringbrunnen höhere Konzentrationen von PFOA, PFOS und GenX im landseitigen Grundwasser festgestellt, was auf eine Quelle im Landesinneren hinweist. Die derzeitige Situation indizierte keinen unmittelbaren Handlungsbedarf für die Trinkwasserversorgung, da die gemessenen Konzentrationen unter dem in der EU-Trinkwasserrichtlinie festgelegten Grenzwert liegen. Künftige gesetzliche Änderungen oder Änderungen bei der industriellen Produktion und der Anwendung von PFAS könnten jedoch zu Problemen führen, da die Ergebnisse darauf hindeuten, dass diese Verbindungen bei der Uferfiltration nicht entfernt werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is an essential source of drinking water globally. Persistent organic pollutants (POPs) have put additional stress on this resource, threaten its quality and raise the need to protect recharge zones and extraction wells in the whole catchment. Meegoda et al. (2020) have classified the primary point sources of PFAS into four categories: industrial facilities, areas utilizing class B firefighting foam, solid waste management facilities, and wastewater treatment facilities. Critical pathways of PFAS to groundwater are through infiltration from the upper unsaturated zone or via bank filtration, which is the focus of this study.
Riverbank filtration (RBF) is widely used as a cost-effective and efficient pre-treatment process for producing drinking water (Tufenkji et al. 2002). Advantages of RBF include the removal to a certain extent of suspended solids, biodegradable compounds, bacteria, viruses and parasites (Hiscock and Grischek 2002). Removal/attenuation of these pollutants can be attributed to one or a combination of several processes, including straining, sorption, colloidal filtration, and microbial degradation (Ray 2011) and can be affected by the travel time and the aquifer hydrogeological properties (Ray et al. 2003). One of the most important fate and transport mechanisms for PFAS is sorption/desorption (Kookana et al. 2022). Studies have shown that soil characteristics (Lyu et al. 2019; Oliver et al. 2019; Qian et al. 2017; Wang et al. 2012), solution chemistry (Aly et al. 2019; Lyu et al. 2019; McKenzie et al. 2015) and compound physiochemical properties (Li et al. 2019; Xiao et al. 2019) can influence the sorption affinity of PFAS to the soil in porous media. Despite RBF’s effectiveness in removing microorganisms, some organic compounds are not eliminated during RBF processes (Heberer 2002). For example, Lange et al. (2007) have detected Perfluorocarboxylic acids (PFCA) and Perfluorosulfonic acids (PFSA) in the groundwater at an RBF site. Unfortunately, there is a lack of studies about the effectiveness of RBF in removing/decreasing PFAS compounds and the governing processes controlling the transport. Hence, there is a need for more studies that can lead to an improved understanding of the fate and transport of PFAS in the subsurface environment as a basis for human health risk assessment.
Different regulations for PFAS have developed over the past 20 years, as summarized by Brennan et al. (2021). The Stockholm Convention on POPs was adopted in 2001 and became international law in 2004. Production of PFOS, one of the original types of PFAS, was prohibited by the convention in 2009. Ten years later, the use of PFOA in firefighting foam and the exception of PFOS in selected applications have been prohibited. In the meantime, however, several other types of PFAS have emerged from industrial processes (e.g. PFHxA, PFNA, PFHxS, GenX). In 2020, the European Food Safety Agency (EFSA) has recommended a tolerable dose of 4.4 ng/kg per week of PFOS, PFOA, PFNA, and PFHxA in food. In the same year, the European Commission published a strategy to ban the production of all PFAS except ones that cannot be replaced. The European drinking water directive (DWD) set the limit for the sum of a specific group of 20 PFAS at 100 ng/l, or, alternatively, set the limit for the sum of all PFAS at 500 ng/l (DWD, DIRECTIVE (EU) 2020/2184). Recently, a draft for a new environmental quality standard directive (EQSD) and a new groundwater directive (GWD) have been proposed by the European Commission where the sum of a specific group of 24 PFAS should be less than 4.4 ng/l PFOA equivalent in ground and surface waters (COM 2022). Studies on the toxicity of PFAS and its human health effects are still ongoing; with increasing knowledge, regulation will change accordingly.
This study aims to serve as a first insight into the behaviour of PFAS during RBF by answering the following questions: (i) Which PFAS compounds are present in various RBF sites along the Danube?, (ii) How are these substances distributed in space?, and, (iii) At which levels do these substances occur compared to two pharmaceuticals and various chemical parameters? In addition to that, this study examines the effect of sorption on PFAS transport.
2 Material and methods
2.1 Study area
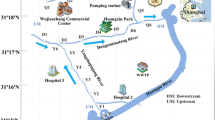
Four transects along the Danube River were selected for monitoring (Fig. 1). Site A is in Vienna, Austria, on the Danube Island, while the rest are on two islands north and south of Budapest, Hungary. Site B1 and B2 are on Szentendre Island, while Site B3 is on Csepel Island. Sites A, B1, and B2 were selected due to the existence of monitoring wells in the transect and in the background water. The groundwater flow is perpendicular to the Danube going inland via different transport routes due to soil heterogeneity.
Map showing the location of the transects where PFAS were monitored, data downloaded from http://tapiquen-sig.jimdo.com and https://www.hydrosheds.org/products
2.1.1 Site A
Site A is characterised by a steady water level in the Danube River due to a dam located 7.5 km downstream of the transect. Furthermore, almost 97% of the banks are clogged, which means most of the water infiltrates from the bottom of the river (Blaschke et al. 2003). The site consists of three monitoring wells with varying distances from 1 up to 30 m away from the riverbank.
2.1.2 Site B1
Site B1 is located on the western side of Szentendre Island. It consists of a horizontal production well which is part of large well group used for drinking water extraction, and three monitoring wells with distances between 15 and 130 m away from the riverbank. The PW is located 56 m away from the riverbank. The water level in the Danube River is very dynamic. There is no evidence of clogging in the riverbank (Nagy-Kovács et al. 2019).
2.1.3 Site B2
Site B2 is on the eastern side of Szentendre Island. It consists of a horizontal production well and five monitoring wells with distances between 5 and 400 m from the riverbank. The PW is located 215 m away from the riverbank. Like Site B1, the water level is also dynamic and the riverbank is not clogged. Danube water was not sampled at this site and results from Site B1 were considered having the same Danube water as at Site B2.
2.1.4 Site B3
Site B3 consists of 30 horizontal production wells. Three of these wells were sampled and then a composite sample was prepared to ensure representative results for larger part of the site. There are no monitoring wells in the area. This site was selected because of a wastewater treatment plant (WWTP) upstream, enabling studying the effects of WWTPs on PFAS concentration in river and groundwater. The transport distance between the riverbank and productions wells is around 90 m.
2.2 Sampling protocol
At Sites B1–B3, samples were taken bimonthly for 1 year between May 2022 and May 2023, while at Site A sampling started six months earlier and samples were collected monthly until March 2022 and then collected bimonthly until March 2023. Samples were collected from the Danube River and the groundwater. Grab samples were taken from the river from approximately 30 cm below the surface using an extension pole with bottle holder. Before groundwater sampling, at least three times the volume of the monitoring well was pumped and discarded to ensure a fresh sample. To do so, a submerged pump was used. The connecting tubes of the pump were made of polypropylene to avoid the risk of sorption to the material. Standard parameters (EC, T, pH and dissolved oxygen (DO)) were measured at each sampling point using a WTW Multi-parameter portable meter. The sample bottles were rinsed three times with sample water before filling. At each site, the samples were collected within one day, starting from the furthest monitoring well from the riverbank and moving towards the Danube River, as the Danube River was assumed to have the highest PFAS concentrations. In between monitoring points, the equipment was flushed with distilled water. For PFAS assays, 1 L bottles made of polypropylene were used for collecting the samples. For chemical assays, 100 ml glass bottles were used: total organic carbon (TOC), Nitrate (NO3), Chloride (Cl) and pharmaceutical analysis (Carbamazepine and Diclofenac). The latter was only performed at the sampling Site A.
On the sampling campaign on 23.03.2023, extra samples were collected to carry out a complete chemical investigation of the major cations, anions and metals for comparing the water types at Sites A, B1, and B2.
2.3 Analytical methods
2.3.1 PFAS and pharmaceuticals
Targeted analysis was performed for 32 different PFAS and 2 pharmaceuticals (Table 1) by using Liquid Chromatography Mass Spectrometry (LC-MS) according to the method provided by EPA (2022). The samples were concentrated using solid phase extraction (SPE). Manual SPE cartridges were used for PFAS, while for the Pharmaceuticals, online SPE with an automatic instrument was used (Stoob et al. 2005).
2.3.2 Chemical parameters
The rest of the chemical parameters were analysed according to the standard drinking water methods. Nitrate was analysed using spectrophotometer for the regular sampling analyses and using ion chromatography for the complete chemical analysis (ISO 10304-1). Chloride was analysed with high-performance liquid chromatography (HPLC) for the regular sampling analyses and using ion chromatography for the complete chemical analysis (ISO 10304-1). All anions and cations for the complete analysis were detected using ion chromatography (ISO 14911, ISO 10304-1). Iron, aluminium, and manganese were analysed with atom absorption spectroscopy (DIN 38406-32, DIN 38406-33, and ISO 12020). TOC was analysed using the TOC analyser Shimadzu TOC‑V and TEKMAR DOHRMAN (EN 1484).
2.4 Quality control
The quality control for PFAS and pharmaceuticals was conducted in two steps. First, to determine the recovery rates of the method, an extracted internal standard (EIS) was added to the process of SPE. Second, to ensure a high-quality concentration calculation, a non-extracted internal standard (NIS) was added to each tube before putting it into the LC-MS. The whole process was conducted in duplicates from the beginning to the end. The recovery rates were within the standard range (80–110%) and the accuracy of the analysis was ±10%. The limit of Quantification (LOQ) was determined by taking 10 times the level of noise in the analysis, which varies per compound.
2.5 Statistical analysis
The statistical analysis was divided into two parts: (i) the processing of censored data and (ii) statistical testing of sampling results. Firstly, the censored data, meaning data with concentrations lower than the limit of quantification (LOQ), were dealt with using the regression on order statistics (ROS) according to Helsel (2011). When any parameter has less than three data points above LOQ in any sampling location, then the LOQ value specific for that parameter was given in place of < LOQ and it was disregarded in the statistical analysis of significant differences. Secondly, a Kruskal-Wallis test (for locations where data from more than two monitoring points are compared) and U‑test (for locations where data from two monitoring points were compared) were performed to test for significant differences between the data (p < 0.05). Correction for the familywise error rate, i.e., false positive rate was performed after the tests to adjust the p‑values following the Benjamini-Hochberg method (Benjamini and Hochberg 1995). The normality of the data was tested with a Shapiro-Wilk test before performing significant differences tests (Gardener 2017). All the analyses were carried out using the R software (R Core Team 2021).
3 Results and discussion
3.1 Analysis of chemical parameters and pharmaceuticals
The results for nitrate, chloride, EC, pH, TOC, and the two pharmaceuticals (Diclofenac and Carbamazepine) are shown in Fig. 2. pH was stable at all sites with an average of 7.6 (not shown). Chloride concentrations were similar between wells at Site A and to some extent at Sites B1 and B3 (Fig. 2). At Site B2, the chloride concentrations were consistently higher by three orders of magnitude in MW5 in the background water, while chloride concentrations in the three monitoring wells (MWs) in the transect between the riverbank and the production well (PW) were similar to the other sites. There was a clear influence of background water on MW4 and PW water quality, where concentrations of all compounds were higher than in the river. EC was persistently higher in the background water than closer to the river, and was higher in Budapest than in Vienna, with highest values measured at Site B2.
Chemical parameters measured throughout the sampling campaign in the Danube, monitoring wells (MWs), and, production wells (PWs) at Sites A, B1–B3. The horizontal line shows the median value, the boxes are the 25th (Q1) and 75th (Q3) percentiles, and the whiskers extend to extreme data points but no more than the ±1.5 interquartile range (IQR). Note that the Y‑axis is freely scaled
NO3-N concentrations were reduced during subsurface passage at Sites A, B1, B3, and in the first three MWs at Site B2, indicating that denitrification processes were taking place. MW5 in the background water at Site B2 showed higher levels of NO3-N than in the rest of the wells, indicating agricultural water seepages or flow paths from other sources of NO3-N towards these MWs. The Danube had generally higher TOC concentrations compared to the groundwater, which can be attributed to filtering of suspended matter during BF. TOC concentrations showed higher variability in Danube water at Site B3 than upstream the Budapest WWTP (Sites A and B1).
Compared to the other chemical parameters, Diclofenac and Carbamazepine behaved differently. Diclofenac showed a significant reduction during infiltration into the riverbank, which is in agreement with the literature (de Carvalho Filho et al. 2022). Carbamazepine was persistent until the furthest MW where concentrations decreased. In the study of van Driezum et al. (2019) which was conducted in a study area at the Danube 16 km downstream of Site A, Carbamazepine was also persistent in groundwater up to a distance of approximately 24 m from the Danube and then decreased.
The complete chemical analysis of the major drinking water parameters showed that the water in the Danube and the groundwater were similar except for the background water at Sites B1 and B2 (MW3 at Site B1, and, MW4 and MW5 at Site B2). At these sites, higher values of NO3, Mg, SO4 and HCO3 were found compared to the Danube or other wells (data not shown). This could be due to the influence of agricultural water seepage or other sources within the Szentendre Island at Budapest. Changes in the Danube water chemistry were minimal between Vienna and Budapest. The error in the electrical balance at all locations was less than 1%, in agreement with the EC measurements. The dominant water type was g2-CaHCO3+, which means fresh water with a moderate alkalinity level, dominated by Ca and HCO3 ions and a surplus of cations.
3.2 PFAS concentrations
The analysis for PFAS targeted 32 different compounds of varying chain length and functional groups. Only 10 compounds had at least three values higher than LOQ during the monitoring period in all transects (Figs. 3 and 4). 6:2 FTS and N‑EtFOSA were detected only at Site A (results not shown). 6:2 FTS was detected there three times in the Danube and with concentrations lower than 2 ng/l, while N‑EtFOSA was only detected once in the Danube and in MW1. All PFAS had concentrations less than 10 ng/l at all sampling sites with only a few exceptions. PFOA had higher concentrations exceeding 10 ng/l at Site B2 in the background water, and NaADONA was the compound with the highest mean concentrations and the largest variability compared to the other PFAS compounds (Fig. 4). Samples from the Danube at Sites A, B1, and B3 did not show significant differences in concentrations, indicating that PFAS originated from upstream sources and there was no large influence from the WWTP in Budapest on concentrations.
At Site A, there was no clear reduction in concentrations during RBF, which could be an indication of minimal sorption of PFAS to the soil during transport. For some compounds, such as PFPeA and PFBA concentrations varied in the MWs along the transects, which may be attributed to mixing due to preferential flow.
Similarly, at Site B1, no clear reduction was found except for PFOA which was marginally reduced between the river and the PW. PFAS concentrations were slightly higher at Site B1 compared to Site A. The concentrations of three PFAS (PFOA, PFOS, and GenX) were higher in the background water (MW3) than in MW1 and MW2, indicating the presence of an inland source of PFAS.
At Site B2, some substances, such as PFBS, PFHpA, and PFHxS, were comparatively stable during subsurface passage similarly as at the other sites. PFOA, PFOS, and GenX showed the same patterns as at Site B1, but with even higher concentrations in the background water, again indicating the presence of an inland source of these PFAS. PFOA had the highest concentration in MW5 of all wells and compounds except for NaADONA, while PFOS and GenX were highest in MW4, displaying the presence of different local sources. Apparently, PW receives considerable water from the background water resulting in higher concentrations of these PFAS (PFOA, PFOS, and GenX) than in the river. In contrast, the concentrations of PFBA, PFPeA, and PFHxA slightly reduced from the Danube towards MW1 and MW2, then slightly increased towards PW, and then reduced again towards MW4 and 5.
PFBA, PFPeA, and PFHxA showed such a behaviour to some extent also at other sites. On top of that, the concentrations of these compounds showed the highest variability among all PFAS. One explanation might be that their shorter chain lengths and carboxylic functional groups make them more soluble in water, or, they could be affected from alternative sources.
At Site B3, the mixed sample of the three PWs was compared to the Danube. PFBA, PFPeA, and PFHxA showed a noticeable reduction in concentration during infiltration into the groundwater, similar as at Site B2, while PFOA and PFHpA were slightly reduced. PFBS and GenX showed no reduction during subsurface (or groundwater) passage, while PFHxA showed a slight increase. As at Sites B1 and B2, PFOS was higher in the PW than in the river. However, this was not the case for PFOA nor GenX, implying that PFOS originates from different sources than the Danube.
NaADONA was presented separately because of its high concentrations and large variation in concentrations (Fig. 4). At Sites A and B1, concentrations were stable during RBF but concentrations were higher at Site B1 than Site A. Site B2 showed an increase in concentrations of NaADONA towards the PW, and a decrease in the background water, with very low concentrations in particular at MW5. At Site B3, NaADONA had a lower concentration in the PWs than in the Danube water. These differences imply that NaADONA was influenced by site specific characteristics more so than other PFAS, and further information about the hydrogeological characteristics and groundwater modelling are needed to better understand the responsible processes, i.e., transport pathways and removal processes.
The high PFAS concentrations in background water at Site B1 and B2 is of interest and needs further investigation in regards to possible sources. Other studies have shown that agricultural products such as biosolids, fertilizers and compost mixed with waste materials containing PFAS could lead to an increase in PFAS concentrations in the soil and adjacent groundwater (Johnson 2022; Röhler et al. 2021).
We expected that PFAS concentrations will reduce with distance, however, our study shows that PFAS concentrations exhibit no or only very limited sorption at the investigated bank filtration sites. Sorption is site specific and is mainly influenced by the presence of organic matter and electrical charge of aquifer grains (Kookana et al. 2022). Xiao et al. (2015) found a reduction from 20000 ng PFOA/l to < 100 ng PFOA/l over a distance of 1.4 km along flow path in groundwater. This was, however, attributed to dispersion and dilution as the soil type was not conducive for PFAS sorption. Moreover, studies on column experiments showed that PFAS experience little sorption to sandy soils with low organic content (Wang et al. 2021). This is also the case for our study sites, where sand is the dominant texture type. Further investigation of sorption parameters with heterogeneous soil column experiments is needed to confirm this hypothesis.
3.3 Statistical tests
The Shapiro-Wilk test for normalities illustrated that the majority of the samples are not normally distributed, also shown by the skewed boxplots. Therefore, the Kruskal-Wallis test was used to test for significant differences between multiple samples. For testing significant differences between two samples the U‑test was used. The null hypothesis (Ho) is that there is no significant difference between the samples. Here the p-value, which is the probability of the measured against the null hypothesis, is compared to the significance level of 0.05. Based on the p-value the null hypothesis can be accepted or rejected (Gardener 2017).
At Site A, B1, and B3, there was no significant difference between the PFAS concentrations in the Danube and the monitoring wells (Table 2), supporting the hypothesis of no sorption processes taking place during bank filtration. At Site B1, although PFOA, PFOS, and GenX concentrations differed between the zone from the Danube towards the PW, and the zone from the PW towards the background water, they were not significantly different overall with p-values slightly above the significance level. At Site B3, there was no significant difference between the samples even for PFPeA and PFHxA, which showed a clear reduction in concentrations, and, PFOS which showed higher concentrations in the PW than in the Danube from the visual inspection of Fig. 4. Additional sources in the Danube in-between the sites can be excluded, as the PFAS concentrations in Danube water between the sites were not significantly different.
At Site B2, there were only two compounds (PFBA and PFBS) which were not significantly different, and which are the shortest chain compounds. PFOA, PFOS, and GenX showed higher concentrations in the background water resulting in significantly differences. The rest of the PFAS were also significantly different at this site. Possibly, these compounds are influenced by preferential flows and slight sorption processes which applies in particular for longer chain compounds, in contrast to shorter chain compounds which are more soluble and can travel through the soil matrix.
3.4 Regulations and health risk assessment
According to the DWD, the sum of 20 PFAS (Table 1) should be less than 100 ng/l to be considered safe for drinking water consumption (DWD, DIRECTIVE (EU) 2020/2184). From the 10 parameters presented in this study with sufficient monitoring results above LOQ, 8 are included in this sum. NaADONA and GenX are not (yet) regulated in the DWD. 9 parameters where analysed but not detected above the LOQ, which impedes quantitative assessment for these parameters. In addition, 3 parameters of the list from the DWD have not been included into the monitoring scheme as an analytical method with sufficient accuracy was not available. The DWD limit was not reached according to our study results. The maximum sum was 24 ng/l, if assuming analytical results < LOQ to be 0 ng/l and neglecting not analysed parameters. Even when considering analytical results < LOQ to be equal to LOQ, the sum of PFAS results in 29 ng/l at maximum. Based on this quality standard, the groundwater at our study sites are in line with drinking water requirements in the current situation.
In a new draft of a proposal by the European Commission concerning the Water Framework Directive (WFD), Groundwater Directive (GWD), and Environmental Quality Standards Directive (EQSD), a standard for groundwater and surface waters is included for a sum of 24 PFAS, and set at 4.4 ng/l PFOA equivalent. All 10 substances with analytical results above LOQ in our study are included in this proposal along with their relative potency factor (RPF) converting them into PFOA equivalent (COM 2022). 9 of the parameters analysed in our study but without sufficient values above LOQ are included into this proposal as well. 5 parameters of the 24 included into this proposal have not been analysed in this study because of missing analytical methods with sufficient accuracy. When applying the RPF in the sum of the 10 PFAS with analytical results above LOQ we found an exceedance of the limit for all sampling locations and sampling dates. When considering the monitoring results below LOQ by setting them equal to LOQ, significantly higher values for PFOA equivalents are reached for the PWs (Fig. 5). Exceedances of these standards can be therefore expected in this case.
Sum of 24 PFAS as PFOA-equivalent in the production wells in Budapest for each sampling event, red line represents the proposed quality standard limit according the drafts of GWD and EQSD (COM 2022). The blue bars only include parameters with monitoring results above LOQ. The orange bars consider monitoring results below LOQ to be equal to the LOQ
4 Conclusion
In this study, the presence of 32 different PFAS was investigated along with their spatial distribution in the Danube and groundwater in Vienna and Budapest. The main outcomes are:
-
The same 10 compounds were detected in all investigated transects, indicating no additional sources between sampling sites, as well as no clear influence from the WWTP in Budapest on PFAS concentrations.
-
At Site A and Site B1 there was no clear reduction in PFAS concentrations during RBF in contrast to Site B2, where there was a compound-specific reduction in concentrations. This can be mainly attributed to longer travel times at Site B2 compared to the other sites allowing for more mixing with ambient groundwater and more time for sorption.
-
PFOS, PFOA, and GenX showed higher concentrations in the background waters at Sites B1 and B2, indicating a potential presence of an inland PFAS source.
-
Carbamazepine showed a similar persistence during subsurface transport as PFAS at short travel distances but was reduced after a 30 m distance. In contrast, Diclofenac showed a clear reduction of concentrations in groundwater during RBF.
-
According to the current drinking water quality standards, PFAS pose no threats for drinking water extractions. However, with the new regulation proposals on the horizon, exceedances of standards in groundwater and surface water would have to be expected.
References
Aly, Y.H. et al. (2019): Enhanced adsorption of perfluoro alkyl substances for in situ remediation. Environ. Sci.-Wat. Res. Technol., 5(11): 1867–1875. https://doi.org/10.1039/c9ew00426b
Benjamini, Y., Hochberg, Y. (1995): Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1): 289–300.
Blaschke, A.P., Steiner, K.H., Schmalfuss, R., Gutknecht, D., Sengschmitt, D. (2003): Clogging processes in hyporheic interstices of an impounded river, the Danube at Vienna, Austria. International Review of Hydrobiology: A Journal Covering all Aspects of Limnology and Marine Biology, 88(3–4): 397–413.
Brennan, N.M., Evans, A.T., Fritz, M.K., Peak, S.A., von Holst, H.E. (2021): Trends in the regulation of per-and polyfluoroalkyl substances (PFAS): a scoping review. International journal of environmental research and public health, 18(20): 10900.
de Carvalho Filho, J.A.A. et al. (2022): Efficiency of the bank filtration technique for diclofenac removal: A review. Environ. Pollut.: 118916. https://doi.org/10.1016/j.envpol.2022.118916
COMMISSION, E. (2022): Proposal for a Directive Of The European Parliament And Of The Council amending Directive 2000/60/EC establishing a framework for Community action in the field of water policy, Directive 2006/118/EC on the protection of groundwater against pollution and deterioration and Directive 2008/105/EC on environmental quality standards in the field of water policy.
Council, E.P. (2020): Directive (EU) 2020/2184 Of The European Parliament And Of The Council of 16 December 2020on the quality of water intended for human consumption.
van Driezum, I.H. et al. (2019): Spatiotemporal resolved sampling for the interpretation of micropollutant removal during riverbank filtration. Science of the Total Environment, 649: 212–223.
EPA, E.P.A. (2022): 3rd Draft Method 1633 Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS.
Gardener, M. (2017): Statistics for ecologists using R and Excel: data collection, exploration, analysis and presentation. Pelagic Publishing.
Heberer, T. (2002): Tracking persistent pharmaceutical residues from municipal sewage to drinking water. Journal of hydrology, 266(3–4): 175–189.
Helsel, D.R. (2011): Statistics for censored environmental data using Minitab and R, 77. John Wiley & Sons.
Hiscock, K.M., Grischek, T. (2002): Attenuation of groundwater pollution by bank filtration. Journal of hydrology, 266(3–4): 139–144.
Johnson, G.R., (2022): PFAS in soil and groundwater following historical land application of biosolids. Water Research, 211: 118035.
Kookana, R.S., Navarro, D.A., Kabiri, S., McLaughlin, M.J. (2022): Key properties governing sorption-desorption behaviour of poly- and perfluoroalkyl substances in saturated and unsaturated soils: a review. Soil Research 61(2): 107–125. https://doi.org/10.1071/SR22183
Lange, F., Wenz, M., Schmidt, C., Brauch, H.-J. (2007): Occurrence of perfluoroalkyl sulfonates and carboxylates in German drinking water sources compared to other countries. Water Sci. Technol., 56(11): 151–158.
Li, F. et al. (2019): Adsorption of perfluorinated acids onto soils: Kinetics, isotherms, and influences of soil properties. Science of the Total Environment, 649: 504–514. https://doi.org/10.1016/j.scitotenv.2018.08.209
Lyu, X. et al. (2019): Transport and retention of perfluorooctanoic acid (PFOA) in natural soils: Importance of soil organic matter and mineral contents, and solution ionic strength. Journal of contaminant hydrology, 225: 103477.
McKenzie, E.R., Siegrist, R.L., McCray, J.E., Higgins, C.P. (2015): Effects of chemical oxidants on perfluoroalkyl acid transport in one-dimensional porous media columns. Environmental science & technology, 49(3): 1681-1689.
Meegoda, J.N., Kewalramani, J.A., Li, B., Marsh, R.W. (2020): A Review of the Applications, Environmental Release, and Remediation Technologies of Per- and Polyfluoroalkyl Substances. International Journal of Environmental Research and Public Health, 17(21): 26. https://doi.org/10.3390/ijerph17218117
Nagy-Kovács, Z. et al. (2019): Water quality changes during riverbank filtration in Budapest, Hungary. Water, 11(2): 302.
Oliver, D.P. et al. (2019): The role of surface charge and pH changes in tropical soils on sorption behaviour of per-and polyfluoroalkyl substances (PFASs). Science of the total environment, 673: 197–206.
Qian, J. et al. (2017): Adsorption of perfluorooctane sulfonate on soils: effects of soil characteristics and phosphate competition. Chemosphere, 168: 1383–1388.
R Core Team (2021): R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org
Ray, C. (2011): Riverbank filtration concepts and applicability to desert environments, Riverbank filtration for water security in desert countries. Springer, pp. 1–4.
Ray, C., Melin, G., Linsky, R.B. (2003): Riverbank filtration: improving source-water quality, 43. Springer Science & Business Media.
Röhler, K., Haluska, A.A., Susset, B., Liu, B., Grathwohl, P. (2021): Long-term behavior of PFAS in contaminated agricultural soils in Germany. Journal of Contaminant Hydrology, 241: 103812.
Stoob, K., Singer, H.P., Goetz, C.W., Ruff, M., Mueller, S.R. (2005): Fully automated online solid phase extraction coupled directly to liquid chromatography-tandem mass spectrometry: Quantification of sulfonamide antibiotics, neutral and acidic pesticides at low concentrations in surface waters. J. Chromatogr. A, 1097(1–2): 138–147.
Tufenkji, N., Ryan, J.N., Elimelech, M. (2002): Peer reviewed: the promise of bank filtration. ACS Publications.
Wang, F., Liu, C., Shih, K. (2012): Adsorption behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on boehmite. Chemosphere, 89(8): 1009-1014.
Wang, Y.K., Khan, N., Huang, D.D., Carroll, K.C., Brusseau, M.L. (2021): Transport of PFOS in aquifer sediment: Transport behavior and a distributed-sorption model. Science of the Total Environment, 779: 8. https://doi.org/10.1016/j.scitotenv.2021.146444
Xiao, F., Simcik, M.F., Halbach, T.R., Gulliver, J.S. (2015): Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a US metropolitan area: migration and implications for human exposure. Water research, 72: 64–74.
Xiao, F., Jin, B.S., Golovko, S.A., Golovko, M.Y., Xing, B.S. (2019): Sorption and Desorption Mechanisms of Cationic and Zwitterionic Per- and Polyfluoroalkyl Substances in Natural Soils: Thermodynamics and Hysteresis. Environmental Science & Technology, 53(20): 11818–11827. https://doi.org/10.1021/acs.est.9b05379
Acknowledgements
This work has been funded by the Vienna Science and Technology Fund (WWTF) [10.47379/ESR20013] and the European Union’s Horizon 2020 research and innovation programme under grant agreement number 101036449. The author would also like to thank the VERBUND Hydro Power GmbH (VHP) for providing access to the sampling location in Vienna.
Funding
Open access funding provided by TU Wien (TUW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obeid, A.A.A., Oudega, T.J., Zoboli, O. et al. The occurrence and persistence of PFAS at riverbank filtration sites in the Upper Danube basin. Österr Wasser- und Abfallw 75, 515–527 (2023). https://doi.org/10.1007/s00506-023-00974-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00506-023-00974-w
Keywords
- PFAS sorption/removal
- Riverbank filtration
- Groundwater transport
- Pharmaceuticals
- Spatial distribution
- Regulations and guidelines