Abstract
Lake Nasser is an artificial reservoir that represents the national freshwater reserve of Egypt and has a number of khors (side extensions) along its eastern and western banks. These khors covered approximately 79% of the lake’s total surface area and have an important effect on water quality and fish production in the lake. This study aimed to monitor and assess the water quality, distribution, and structure of the biotic community, including phytoplankton, zooplankton, macrophytes, epiphytes, and macrobenthos in two main khors (Tushka West and Dahmeit) during postflood and preflood periods. There was urgency associated with the study, as it was the last opportunity before the storing of the water behind the Grand Ethiopian Renaissance Dam (GERD), which had begun to fill in July 2020. Although the study confirmed that the water quality of Lake Nasser was good, it is classified as a eutrophic lake (depending on the levels of Chlorophyll-a and nutrients). This study found that pH, NO2, SiO4, NH4, PO4, and temperature were the factors with the greatest effect on the biota community, especially in Dahmeit Khor. In contrast to the phytoplankton and macrobenthos, the highest total density of zooplankton and epiphytic microalgae were detected during the postflood period. This study could be useful in monitoring possible changes in the Lake Nasser environment after the complete filling of the GERD reservoir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lake Nasser is a relatively closed ecosystem with very irregular shoreline characterized by the presence of 85 inundated valleys called khors (The habitats for fish breeding and feeding). There are approximately 48 khor on the eastern side and 37 on the western side of the lake. Khors which have perimeters of more than 100 km at a water level of 180 m MSL include: Kalabsha, Wadi El-Allaqi, Kurkur, Korosko, Khor El-Birba (El-Ramla), Rahma, Dahmeit, Shaturma, Wadi Abyad, Mariya Masmas, Tushka, and Or (Latif 1984). According to Goher and Ali (2009), the total length of the khor systems when the lake is full, i.e., at water level 180 m MSL, is nearly 3,000 km. The total surface area of the khors is 4,900 km2 (79% of total lake surface). In volume, they contain 86.4 km3 of water (55% of the total lake volume). The khors are the main source of fish caught in the lake (about 90%) (Goher et al. 2021b). Some khors represent auxiliary, semiisolated lakes. Allaqi, Kalabsha, and Toushka Khors are the largest, while others like Korosko and El-Sadake are relatively narrow. Lake Nasser and its Khors are vital participants in the water resources and economy in Egypt, therefore, the regular studies of the environmental status, aquatic communities is of great importance for the full assessment of the complete environmental situation of the Lake Nasser and its khors (e.g. Samaan and Gaber 1976; Latif 1984; Abd El-Monem 1995; Taha and Mageed 2002; El-Shabrawy and Dumont 2003; Ali and Soltan 2006; Goher and Ali 2009; El-Serafy et al. 2009; El-Enany 2009;; Abd El-Karim 2014; Hussian et al. 2015; Khalifa et al. 2015; Abdel Gawad and Abdel-Aal 2018; Rizk et al. 2020; Shalloof et al. 2020; Goher et al. 2021b; Haroon and Abd Ellah 2021; Nassif 2021; Zaher and Aly 2021).
The water resources of Egypt are very limited, therefore any future activities and perturbations on the lake due to the construction of the Grand Ethiopian Renaissance Dam (GERD), which began filling in July 2020, should be carefully considered before implementation. In context, the current study aims to evaluate the water quality, structure and distribution of biotic communities in Tushka West and Dahmeit Khors of Lake Nasser during postflood (November 25–30, 2019) and preflood (June 22–27, 2020) as the last opportunity before the storing of water behind the GERD. By recording the ecological status of Nasser Lake before water storage by the GERD reservoir commences, this study should be useful in monitoring its overall ecological status. Additionally, this study enables the monitoring of possible changes in the lake’s environment and ecology which may result from effects of the GERD.
2 Materials and methods
2.1 Study area
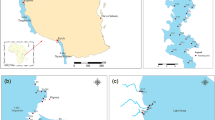
Water samples were collected from five sites each in Tushka West and Dahmeit Khors during the postflood 2019 (November) and preflood 2020 (June) periods (Table 1; Fig. 1). Those two khors were chosen to represent the different khor types of the lake. Tushka West Khor is one of the largest Lake Nasser khors, contains 17 islets, some of which are usually fully exposed and some others which are covered by water during high flood periods. It is located in the south-west of the lake between 22°31′41.3″ and 22°43′27.5″ N latitude, and 31°32′19.7″ and 31°47′08.7″ E longitude, and is separated from the main channel of the lake with a wide natural water channel of 11.25 km width with no outlet. It extends for 34.5 km in an east–west direction, with a maximum width of 12.7 km, total surface area of 184.8 km2, regular bottom topography, and medium water depth (from a few centimeters to ≤ 30 m). The shallower water depths are primarily found close to the shoreline and along the western bank of the khor; however the deeper depths exist near the inlet of the khor (Abd ellah and El-Geziry 2016). Meanwhile, Dahmeit Khor is smaller compared to Tushka West Khor, and is located in the north-eastern corner of the lake and includes 10 islets. This khor occupies the area between 23°43′21.4″ and 23°48′01.4″ N latitude, 32°57′22″ and 33°03′17.6″ E longitude, and is connected to the main channel of Lake Nasser by a natural narrow mouth (0.45 km width) with no outlet. This khor spreads in the east–west direction, with a total length of 12.4 km, a maximum width of 0.9 km, and a surface area of 12.0 km2. This khor is classified as a deep water khor with no regular bottom topography, and has depths which vary between a few centimeters and 55 m. The greatest water depths are found near the entrance of the khor, however the shallowest depths occur near the shoreline and along the eastern side of the Khor.
The map of locations and the sampling sites in Nasser Lake khors (after Abdel Gawad and Abdel-Aal, 2018)
2.2 Sampling program
2.2.1 Abiotic characteristics
Subsurface and bottom water samples were collected using polyvinyl chloride water samplers (2L). Glass stoppered oxygen bottles were used for measurements of dissolved oxygen (DO) and biochemical oxygen demand (BOD), and were carefully filled with water samples. Water temperature (°C), pH, and conductivity (EC, µS cm−1) were measured in-situ using a Hydrolab multiparameter (Orion Research Ion Analyzer 399A, Orion Research, Cambridge, MA, USA). Water transparency was measured using a white/black Secchi disk (25 cm in diameter). Total solids (TS), Total dissolved solids (TDS), Total suspended solids (TSS), Water Alkalinity, Chlorides (Cl−), DO, BOD, Chemical Oxygen Demand (COD), Nitrogen–Ammonium (N–NH4), Nitrogen–Nitrite (N–NO2), Nitrogen–Nitrate (N–NO3), Orthophosphates (P–PO4), Silicates (SiO4), Calcium (Ca2+), magnesium (Mg2+), sodium (Na+), and potassium (K+) levels were determined using the methods discussed by the American Public Health Association (APHA 2005).
2.2.2 Biotic characteristics
Planktons (phytoplankton and zooplankton), macrophytes, and attached epiphytic microalgae were collected, prepared, and preserved according to the methods described by Goher et al. (2021b). The macrophyte samples were identified according to Boulos (2005). The phytoplankton and epiphytic microalgae species were identified according to previous studies (Cleve-Euler 1952; Prescott 1978; Wehr and Sheath 2003; Komárek and Zapomělová 2007; Taylor et al. 2007). The zooplankton species were identified according to previous studies (Edmondson 1959; Pennak 1978; Verheye and Dumont 1984). The macrobenthos samples were collected using an Ekman grab sampler (opening area of 750 cm2). Three grab samples were taken from each station at the upper layer of the bottom sediments. The samples were immediately washed to remove adhering sediments, sieved through a 500 μm mesh diameter net, and preserved with 10% formalin. In the laboratory, the samples were washed and sieved through a 500 μm mesh diameter net and preserved with 7% formalin. The benthic animals were identified according to previous studies (Wirth and Stone 1956; Habashi 1993; Brinkhurst and Jamison 1971; Brown 1980; Madden 2010).
2.3 Statistical analyses
The summary statistics of data obtained (minimum, maximum, mean) and correlation (predictive statistics) were carried out using the XLSTAT 2013 add-on for Microsoft Excel. The correlation coefficients were considered significant at the 95% confidence level (p ≤ 0.05). The percentage of the coefficient of variability (CV) was calculated using Microsoft excel program 2019.The multivariate analysis method of Canonical Correspondence Analysis (CCA) was performed using the CANOCO v. 4.0 program (Ter Braak and Smilauer 1998). This elucidated the relationships between the water characteristics and the aquatic organisms within different sampling stations in Tushka and Dahmeit Khors during post and preflood periods.
3 Results and discussion
3.1 Water characteristics
The physicochemical characteristics of water in Tushka and Dahmeit Khors are shown in Tables 2 and 3. Temperatures fell within the expected range according to the period of sampling, and varied from 20.8 °C for the bottom water at Dahmeit Khor during the preflood period, and 30.6 °C for surface water of Tushka Khor, during the same season. ANOVA data showed a highly significant difference (p < 0.001) between the preflood and postflood periods, and a remarkable difference (p < 0.05) between the surface and bottom water. In general, the water temperature was suitable for fish growth (Talab et al. 2016; Goher et al. 2019). Secchi disc measurements (transparency) reflected the clarity of the water column and the penetration of the light to the deeper layers (Goher et al. 2015, 2018). The decrease in water clarity at Tushka Khor (250 cm, on average) compared to Dahmeit Khor (368 cm, on average) was obvious. An opposite result was observed for TSS, which increased in Tushka Khor in the south of the lake. These results may be related to the velocity of the water current, which is reduced northward where Lake Nasser is characterized by riverine and lacustrine behavior in the southern and northern sections, respectively (Goher et al. 2021b; Hassan et al. 2021). In the same context, EC and TDS notably increased in the northern Dahmeit Khor, especially during the postflood period. This was likely because the flood water was loaded with different ions, with a temporal difference (p < 0.001) and CV values of 12.47% and 6.43% during postflood and preflood, respectively. In general, the water of Lake Nasser khors was classified as having low EC and TDS in comparison to Nile River water downstream of the High Dam. These findings were in harmony with the findings of Imam et al. (2020).
pH is one of the most important parameters in water quality. It affects most biological and chemical processes, and determines the solubility and biology of chemical constituents such as nutrients and heavy metals (Goher et al. 2021a; USGS 2021). Concerning the results obtained, the water of the Lake Nasser khors were alkaline and varied between 7.69–8.85 and 7.7–8.92, with CV values of 4.29% and 4.71% during the postflood and preflood periods, respectively. It is worth mentioning that the pH values were within the national and global acceptable limits (6.5–9) and are suitable for usages such as drinking, irrigation, and aquatic organisms’ environment. This result is in agreement with those obtained by Rizk et al (2020), Imam et al. (2020) and Goher et al. (2021b) for Lake Nasser, and El Sayed et al. (2020) for the Nile River.
DO is the most significant factor in the aquatic ecosystem. It is a critical component not only for plant and animal survival but also for water quality and chemical reactions within water bodies (Sensorex 2021). The water of studied khors of Lake Nasser was oxygenated during the study periods. The average values of DO recorded were 6.59 and 6.09 mg/L, with CV values of 26.94% and 19.03%, respectively, during the postflood and preflood periods. The slight decrease of DO values during the preflood period was attributed to the elevation of water temperature that reduced the solubility of oxygen in the water (Goher et al. 2019; Hassouna et al. 2019). The BOD and COD, which are functions of organic water pollution (Goher et al. 2014), were at low levels of 3.15 and 1.91 mg/L, on average, with CV values of 11.06% and 3.54% for BOD during the postflood and preflood periods, respectively; and 5.3 and 4.75 mg/L, on average, with CV values of 27.11% and 39.37% for COD, respectively, during the postflood and preflood periods. Based on the general average of BOD and COD in Dahmeit khor (2.35 and 5.18 mg/l, respectively) and Tushka khor (2.72 and 4.86 mg/l, respectively), the results obtained revealed that the water of the khors of Lake Nasser was not contaminated with organic pollutants, and they were, in general, below the standard levels of 3 and 10 mg/L for BOD and COD, respectively. However, the relative increase of BOD during the postflood period may be attributed to the abundance of microorganisms in Lake Nasser water after flooding, as reported by Goher et al. (2021b).
The basic nutrient salts, except PO4, increased in the postflood period with a highly significant temporal difference (p < 0.001) between postflood and preflood periods which coincides with those obtained by Goher et al. (2021b). Generally, ammonia and nitrate were seen to fluctuate widely, with ranges of 39.44–867.0 µg/L and 18.4–803.2 µg/L, respectively, with a high coefficient of variations of 88.1% and 55.67% for NH4 and 103.46%, and 68.17% for nitrate in postflood and preflood periods, respectively. In contrast, low levels of NO2 and PO4 were observed with average values of 12.62 and 8.58 µg/L, and 9.52 and 22.1 µg/L in postflood and preflood periods, respectively, with CV values of 34.74% and 82.11% for NO2, and 72.59% and 29.44% for PO4.
Similar to the observed distribution pattern of nutrient salts, the major ions, except SO4, recorded higher values after flooding. The average values were 108.27 and 98.45 for HCO3, 9.83 and 9.39 for Cl, 14.28 and 13.37 for Ca, 9.23 and 8.47 for Mg, 13.34 and 11.71 for Na, and 4.44 and 4.14 mg/L for K, on average, in postflood and preflood periods, respectively. In turn, SO4 showed higher values during the preflood period, recording 6.14 mg/L on average, compared to 5.91 mg/L during the postflood period. Anions and cations, except CO3, had low CV values ranging between 2.74% for Mg during the preflood period, and 10.78% for Na during the postflood period. Most anions and cations were highly positively correlated with EC and TDS (r = 0.84–0.94, n = 40; p < 0.001). In contrast, they showed a significantly negative correlation with temperature (r = −0.53 to − 0.74, n = 40; p < 0.001). The levels of the major anions and cations in the khor water do not cause any adverse, negative, or harmful effects on potability (EWQS 2007; USEPA 2018; WHO 2017), agriculture according to FAO (Ayers and Westcot 1985), or aquatic organisms (USEPA 2021; CCME 2007).
In addation to transperancy, EC and TDS; as mentioned above; pH and DO showed a significantly high difference between Dahmeit and Tushka khors (p < 0.001), while ANOVA Data of COD recorded a significant difference (p < 0.05). The average values of pH, DO, COD, HCO3 and Na were (8.25 and 8.77; CV 4.48 and 1.48%), (5.51 and 7.27 mg/l; CV 26.4 and 14.17%), (5.18 and 4.86 mg/l; CV 12.46 and 5.1%), (111.6 and 95.15 mg/l; CV 8.39 and 5.02%) and (13.52 and 11.52 mg/l; CV 9.59 and 6.45%) in Dahmeit khor and Tushka khor, respectively. On the contrary the other parameters such as NH4, NO3, NO2, PO4, SiO2, CO3, Cl, Ca and Mg did not show any significant difference.
In general, water quality refers to specific desired levels of the chemical, physical, and biological characteristics of water, based on identified uses for aspects of life (UNP 1997). Accordingly, the hydrographic characteristics and chemical constitutes of Lake Nasser khors were within the national (EWQS 2007) and global (USEPA 2018, 2021; WHO 2007; Ayers and Westcot 1985; CCME 2007) permissible levels for the identified uses. This finding confirms the good quality of Lake Nasser water and establishes that the water of Lake Nasser is considered a blank site (standard sample) for any change or contamination of the water downstream of the High Dam. Additionally, the most effective factor for altering the measured parameters was flooding, where most of the water constituents showed a significantly high temporal difference (p < 0.001), except for DO, which recorded similar levels during both periods. In contrast, most parameters, except temperature and pH values, did not show remarkable variations between surface and bottom water. It is worth mentioning that there were highly significant differences (p < 0.001) for most parameters between Dahmeit Khor and Tushka Khor.
3.2 Assemblages of aquatic organisms
In a complex natural environment, such as Lake Nasser, where several factors operate simultaneously, it is not easy to generalize and designate some factors as being more important than others. The yearly flood of the Nile, which occurs in August and originates from the Ethiopian Highlands is the most important factor affecting the ecosystem of Lake Nasser, and hence it may affect the temporal distribution, abundance, and community composition of organisms in the lake (e.g., planktonic algae, epiphytic algae, zooplankton, macroinvertebrates, etc.) (Samaan and Gaber 1976; El-Serafy et al. 2009; Abdel Gawad and Abdel-Aal 2018; Goher et al. 2021b). The changes in the density of the aquatic organisms (Fig. 2), and the macrophyte abundance in Lake Nasser khors (Tushka and Dahmeit) during the flood cycle could be summarized as follows:
3.2.1 Planktons
The density and composition of phytoplankton can indicate the trophic status and affect the food web structure in aquatic ecosystems (Jeffrey and Vesk 1997; Leterme et al. 2006). The associations between phytoplankton and zooplankton are well known. Specifically, the distribution and biomass of phytoplankton is not solely affected by the physicochemical properties of water, but grazing by zooplankton is also an important factor (Kawecka and Eloranta 1994; Burkepile and Hay 2006; Pascal and Fleeger 2013; Yuan and Pollard 2018). This concept is established in the present study by the negative relationship between those two groups, as shown in Fig. 2A, B.
The phytoplankton assemblages observed in Tushka and Dahmeit Khors were comprised of seven phyla: Cyanobacteria, Bacillariophyta, Charophyta (planktonic), Chlorophyta, Cryptophyta, Miozoa, and Ochrophyta. The Ochrophyta was only recorded during the postflood period and with only one species. In general, the density in the preflood period was higher than in the postflood period, and extended over threefold higher at sites T3 and T5 (Fig. 2 A&B), which may have contributed to the N/P ratio in the preflood period (~ 6 to 17), and the high temperatures during summer. Redfield (1958), Goldman et al. (1979), and Harris (1986) considered the atomic N/P ratio for phytoplankton to be approximately 16, and Trombetta et al. (2019) indicated that high temperatures induced phytoplankton growth. The total density in Tushka Khor ranged from 201.6 to 403.7 × 104 cells/L−1 during the postflood period (Fig. 2A), and from 448.8 to 1378.3 × 104 cells/L−1 during the preflood period (Fig. 2B). The highest density was found at site T3 during both the post and preflood periods. In Dahmeit Khor, the total density changed in the postflood period from 174.5 to 247.6 × 104 cells/L−1 (Fig. 2A), and in the preflood period from 211.4 to 323.7 × 104 cells/L−1 (Fig. 2B). These results are confirmed by the higher average Chlorophyll-a values found during the preflood period (171.11 µg/L−1), compared to 58.22 µg/L−1 during the postflood period. The highly positive correlation of Chlorophyll-a with PO4 (r = 0.59, n = 40; p < 0.001) may indicate that phosphorus was the limiting factor. This finding was confirmed by the N/P ratio (> 16, on average). Koerselman and Meuleman (1996) stated that nitrogen is the limiting factor at N/P < 14, and that at N/P ratios between 14 and 16 either N or P can be limiting, while N/P > 16 indicates that phosphorus is the limiting factor. It is noteworthy that on the basis of nutrients content (Tables 2 and 3), Lake Nasser khors are considered eutrophic, and the levels of Chlorophyll-a found supported this observation and were in harmony with the findings of Hussian et al. (2015) and Hussian and Haroon (2019). According to Carlson and Simpson (1996) the eutrophic lakes contain a level of Ch-a and TP of more than 7.3 µg/l and 24 µg/l, respectively which is lower than the recorded levels in the studied area. Where, the Ch-a and TP contents in the two khors excess these values, recording 126.56 and 102.76 µg/l (on average) and 42.1 and 42.44 µg/l (on average) in Dahmeit Khor and Tushka Khor, respectively.
Generally, the structure of phytoplankton in the two khors was similar. Cyanobacteria (22 spp.) were seen to dominate the phytoplankton assemblages by 69–77% and 80–92% of the total density in Tushka Khor, and by 73–79% and 66–89% in Dahmeit Khor, in postflood and preflood periods, respectively. The second most dominant group was the chlorophytes (51 spp.) making up 8–13.3% and 6–14% of the samples in Tushka, and 9–13.6% and 5.6–32% in Dahmeit during the postflood and preflood periods, respectively. Meanwhile, the diatom (31 spp.) share of the phytoplankton assemblages were 11–15% and 1.5–8.5% in Tushka Khor, and 10.8–12.5% and 2.5–7% in Dahmeit during the postflood and preflood periods, respectively.
During the postflood period, the highest densities of phytoplankton were found among Chroococcus disperses, Chroococcus minimus, Cylindrospermopsis raciborskii, Merismopedia tenuissima, and a Pseudoanabaena sp., comprising 0.6–64% of the total density. Meanwhile, during the preflood period, the phytoplankton community was dominated by Aphanocapsa elachista var. conferta, C. minimus, Chroococcus minutus, C. raciborskii, M. tenuissima, Oscillatoria tenuis var. tergestina, Chlamydomonas globosa, Scendesmus bijugatus var. alterans, and a Rhodomonas sp., comprising 0–78% of the total density. These results were in agreement with previous findings of phytoplankton assemblages in Lake Nasser, and Egyptian ecosystems in general (e.g., Samaan and Gaber 1976; Latif 1984; Abd El-Monem 1995; Taha and Mageed 2002; Abdel-Hamid et al. 2014; Abd El-Karim 2014; Hussian et al. 2015; Abdel-Hamid et al. 2017; Abdel Gawad and Abdel-Aal 2018; Abdel-Aal 2021; Goher et al. 2021b; Zaher and Aly 2021).
The zooplankton assemblages observed in Dahmeit and Tushka Khors during the post and preflood periods were represented by twenty four species in three phyla: Copepod (2 spp.), Cladocera (6 spp.), and Rotifer (16 spp.). The total zooplankton density ranged from 30 to 69 ind. L−1, and from 43 to 92 ind. L−1, in Tushka and Dahmeit, respectively in the postflood period (Fig. 2A). In the preflood period, the total density was generally lower and ranged from 20 to 97 ind. L−1 in Tushka, and 22 to 56 ind. L−1 in Dahmeit Khor (Fig. 2B). A noticeable increase was observed in downstream total zooplankton during the postflood period in both Tushka and Dahmeit Khors, and in preflood Dahmeit Khor (Fig. 2A&B). This observation may be due to the addition of high plankton densities resulting from the flood (Parmar et al. 2016). On the contrary, the downstream zooplankton density was decreased during the preflood season at Tushka (Fig. 2B), which is due to the movement of zooplankton away from the hot surface water during the summer (Goher et al. 2021b).
Generally, the relations in zooplankton structure consisted of Copepoda > Cladocera > Rotifera. In Tushka Khor, the average share of these phyla relative to the total density amounted to 72%, 14%, and 14% during the postflood period, whereas during the preflood period, it amounted to 59.15%, 22.7%, and 18.14%, respectively. While in Dahmeit Khor, these phyla amounted to 81.75%, 12.5%, 5.7%, and 56%, 16.17%, 27.69% during post and preflood periods, respectively. The presence and dominance of zooplankton species were spatial, where Asplanchna priodonta, Ceirodaphnia cornuta, and Collotheca ornate were the dominant species at Dahmeit Khor, comprising 0–9.7% and 0–23.4% of the total densities during the postflood and preflood periods, respectively. While, Daphnia longispina, Diaphanosoma excisum, and Keratella cochlearis were the dominant species at Tushka Khor, comprising 0–11.1% and 0–27.6% of the total densities during the postflood and preflood periods, respectively. Nauplius larvae was the most dominant form of copepod in both khors, with a high density ranging from 35.7% to 78.7% of total zooplankton density during the postflood period, and from 28 to 47% during the preflood period. These results agreed with the findings of El-Shabrawy and Dumont (2003), El-Serafy et al. (2009), Khalifa et al. (2015) and Goher et al. (2021b). Additionally, El-Enany (2009) found copepods comprised 68.8% of the total zooplankton in six khors of Lake Nasser. The dominance of copepods in Lake Nasser khors is due to the abundance of nauplius larvae which feed primarily on phytoplankton.
3.2.2 Macrophytes and attached epiphytic microalgae
The study of the diversity and distribution of aquatic macrophytes in a lake can provide crucial information about its productivity. These plants were recorded as an important component of the aquatic ecosystem, not only as a food source for aquatic invertebrates and fish (Sharshar and Haroon 2009) but also because they act as an efficient accumulator of nutrient elements and heavy metals (Elmorsi et al. 2019) which helps improve water quality. Additionally, they impact the growth, distribution, and diversity of other aquatic organisms which have an effect on the functioning of wetland ecosystems (Haroon et al. 2020; Othman and Haroon 2020; Tahoun et. al. 2021). The history of the diversity and distribution of Lake Nasser macrophytes was discussed by Haroon and Abd Ellah (2021). They detected only three submerged macrophytes species (Myriophyllum spicatum, Potamogeton schweinfurthii, and Najas marina) at different sites in the lake throughout the period from 2013 to 2020, but these had a major impact on the lake ecosystem as mentioned by Ali and Soltan (2006).
During the present study period, only two submerged macrophytes species from two genera (Myriophyllum spicatum L., Potamogeton schweinfurthii A. Benn) were detected in the study area. During the winter M. spicatum L. (Haloragaceae) was found to be the most dominant and widely distributed, and was present in 100% of the sampling sites. In contrast, P. schweinfurthii A. Benn (Potamogetonaceae) was very rare and was recorded at only one site, T1. During the spring M. spicatum L was the only recorded macrophyte species at the study site, which indicates the adaptability of this invasive species and its ability to thrive under different environmental conditions.
Many studies have stated the important role of epiphytic algae in primary productivity (e.g., Kupferberg 2003; Adam et al. 2017) and enhancement of macrophyte growth by providing nutrients via decomposition processes (Carvalho et al. 2015). However, the abundance of macrophytes may affect microalgae growth through allelopathic compounds (Körner 2006; Haroon and Abdel-Aal 2016). Generally, the epiphytes assemblages in Tushka and Dahmeit Khors comprised of four phyla: Cyanobacteria (25 spp.), Bacillariophyta (57 spp.), Chlorophyta (38 spp.), and Miozoa (5 spp.). The relation of epiphytic microalgae structure in both khors reversed between the two periods. In the postflood period, the shares of different groups in Tushka were Bacillariophyta (47–75%), Chlorophyta (19–42%), Cyanobacteria (5–10%), and Miozoa (0.1–0.7%), while the shares in Dahmeit were Chlorophyta (53–63%), Bacillariophyta (19–40%), Cyanobacteria (4–18%), and Miozoa (1–8%). Meanwhile, the shares of the different groups during the preflood period were Chlorophyta (71–74%), Bacillariophyta (19–25%), Cyanobacteria (1–4%), and Miozoa (0.1–0.7%) in Tushka Khor, and Bacillariophyta (47–58%), Chlorophyta (14–33%), Cyanobacteria (12–22%), and Miozoa (3–6%) in Dahmeit Khor. Other studies noticed the dominance of bacillariophyceae (e.g. Corlett and Jones 2007; Abdel-Hamid et al. 2017; Goher et al. 2021b), as diatoms stick to the macropytes by producing extracellular polymeric substances (Tiffany 2011). The importance of bacillariophyceae is due to their use as bioindicators for the ecological status of aquatic environments (Pouličkowá et al. 2004). The total density of epiphytic algae observed during the postflood period ranged from 59.9 to 236.2 × 104 Cells/g−1 of macrophytes in Tushka Khor, and from 46.9 to 248.5 × 104 Cells/g−1 of macrophytes in Dahmeit Khor (Fig. 2A). The highest densities were found at sites T1 and D5. During the preflood period, the total macrophyte density was lower and changed in Tushka from 20 to 60.9 × 104, as well as in Dahmeit which changed from 16.4 to 44.7 × 104 Cells/g−1 (Fig. 2B). The high concentrations of DO (8.20 mg/L), nitrates (387.36 µg/L), and silicates (7.65 mg/L) (Tables 3) during the postflood period may reveal the increasing density of epiphytic microalgae in Tushka Khor (Fig. 2A). The highest densities of epiphytic microalgae species were recorded for Lyngbya limnetica Lem., Dictosphaerium pulchellum Wood, Oscillatoria perornata Skuja, a Mougeotia sp., an Oedogonium sp., Scenedesmus caudatus Corda, Cyclotella ocellata Pant, Gomphonema ventricosum W. Gregory, Melosira granulata (Her.) Ralfs, Navicula cryptocephala Kutz, Neidium dubium (Ehenberg) Cleve, and Syndra ulna (Nitzsch) which were at both khors and comprising 0–48.8% of the total density during the preflood period and 0.0–35.4% of the total density during the postflood period. The dominance of these epiphytes was confirmed by several previous studies (e.g., Hassan et al. 2014; Haroon et al. 2020; Abdel-Aal 2021).
3.2.3 Macrobenthic invertebrates
The benthic invertebrates in Lake Nasser khors exhibited marked spatial and seasonal variations. This was attributed to variations in the prevailing physicochemical conditions and their biological productivity. Twelve macrobenthic invertebrates species were identified in Dahmeit and Tushka Khors during the study period and were represented by four main phyla; Annelida (four spp.), Arthropoda (five spp.), Mollusca (two spp.) and Nematode (one sp.). Annelida was the dominant phylum during the present study and represented 70% by the number of the total macrobenthic fauna at Nasser Khors, followed by Mollusca (19%), Arthropoda (10%), and then Nematode which represented 1% of the total macrobenthic fauna at Lake Nasser khors. These results were in accordance to Wissa (2002), Sleem and Hassan (2010) and Khalifa and Bendary (2016) who recorded that Annelida and Mollusca were the main components of benthic fauna in the Nile River. This macrobenthic decline may be attributed to the nature of the bottom sediments and the availability of food sources, as they were considered the most significant factors determining macrobenthos distribution (Iskaros and El-Dardir 2010).
Generally, the highest densities of macrobenthos at Dahmeit and Tushka Khors were recorded during the preflood period (Fig. 2), which is in agreement with the findings of Abdel Gawad and Abdel-Aal (2018). The population density of the macrobenthic fauna in Tushka Khor ranged from 293 to 1267 ind/m−2 during the postflood period (Fig. 2A), and from 150 to 3125 ind/m−2 during the preflood period (Fig. 2B). The highest two peaks were at stations T1 (3125 ind/m−2; preflood) and T4 (1347 ind/m−2; postflood), due to the intensive number and dominance of Annelida and Mollusca, which may be attributed to the high DO concentrations (6.74 and 8.84 mg/L−1), which causes flourishing of benthic communities, especially oligochaeta, according to Slavevska-Stamenkovć et al. (2010). Meanwhile, in Dahmeit Khor, the population density of the macrobenthic fauna ranged from 347 to 1013 ind/m−2 during the postflood period (Fig. 2A), and from 450 to 1350 ind/m−2 during the preflood period (Fig. 2B). The predominance of oligochaetes in the lake is possibly due to their ability to adapt to various habitats and to their tolerance of low oxygen or anoxic conditions. The dominance of oligochaetes seen in this study is in accordance with the findings of Abdel Gawad and Abdel-Aal (2018). During this study, Limnodrilus udekemianus was ranked as the highest percentage of total macrobenthic density, consisting of 34% and 68.3% in Tushka Khor, and 88.9% and 89.2% in Dahmeit Khor, during preflood and postflood periods, respectively. These findings were in agreement with Wahab et al. (2018).
3.3 Relation between the water variables and aquatic organisms
CCA identified various relationships between different organisms and water physicochemical characteristics, which varied according to sampling site and organism (Figs. 3 & 4). For example at Tushka Khor, the temperature had strong positive associations with Chlorophyll-a, total epiphytic microalgae, Cladocera, and Annelida (r = 0.964, 0.749, 0.583 and.758, respectively) during the preflood period, while during the postflood period it showed a positive association with only Cladocera (r = 0.57), and a negative association with Copepoda (r = −0.72). For samples collected from Dahmeit Khor during the postflood period; pH, NO2, SiO2, NH4, PO4, and temperature were found to be the most effected factors, followed by TDS, BOD, and COD. For example, pH was positively correlated with total phytoplankton count, Cryptophyta (phytoplankton), Cladocera, Total epiphytic microalgae, E-Epi, Total Macrobenthos count, and Nematoda (r = 0.51, 0.69, 0.62, 0.6, 0.6, 0.95, and 0.72, respectively), however it was negatively correlated with Cyanophyta, epiphytic microalgae, Copepoda, Cryptophyta (phytoplankton), Bacillariophyta (phytoplankton) (r = −0.96, − 0.52, − 0.89, and − 0.79 respectively).
Canonical Correspondence Analysis (CCA) joint plot ordination diagram for the abiotic variables (arrows) with the biotic variables (points) during the postflood (A = Dahmeit Khor and B = Tushka Khor). The biotic variables are abbreviated as follow: Chlorophyll a (Chl a), Total Phytoplankton count (T.Ph), Cyanophyta of phytoplankton (Cy-Ph), Chlorophyta of phytoplankton (Ch-Ph), Bacillariophyta of phytoplankton (B-Ph), Dinophyta of phytoplankton (D-Ph), Cryptophyta of phytoplankton (Cp-Ph), Total epiphytic microalgae count (T.Epi), Cyanophyta of epiphytic microalgae (Cy-Epi), Chlorophyta of epiphytic microalgae (Ch-Epi), Bacillariophyta of epiphytic microalgae (B-Epi), Dinophyta of epiphytic microalgae (D-Epi), Total zooplankton count (T.Zoo), Copepoda (Cope), Cladocera (Clad), Rotifera (Rot), Total Macrobenthos count (T.M.b), Annelida (Anld), Arthropoda (Arth), Mollusca (Mols) and Nematoda (Nem)
Canonical Correspondence Analysis (CCA) joint plot ordination diagram for the abiotic variables (arrows) with the biotic variables (points) during the preflood (A = Dahmeit Khor and B = Tushka Khor). The biotic variables are abbreviated as follow: Chlorophyll a (Chl a), Total Phytoplankton count (T.Ph), Cyanophyta of phytoplankton (Cy-Ph), Chlorophyta of phytoplankton (Ch-Ph), Bacillariophyta of phytoplankton (B-Ph), Dinophyta of phytoplankton (D-Ph), Cryptophyta of phytoplankton (Cp-Ph), Total epiphytic microalgae count (T.Epi), Cyanophyta of epiphytic microalgae (Cy-Epi), Chlorophyta of epiphytic microalgae (Ch-Epi), Bacillariophyta of epiphytic microalgae (B-Epi), Dinophyta of epiphytic microalgae (D-Epi), Total zooplankton count (T.Zoo), Copepoda (Cope), Cladocera (Clad), Rotifera (Rot), Total Macrobenthos count (T.M.b), Annelida (Anld), Arthropoda (Arth) and Mollusca (Mols)
Different relations were detected between different recorded organisms. For example: the total phytoplankton count was positively correlated with Rotifera, indicating that phytoplankton abundance can be stimulated by zooplankton, as recorded by Gołdyn and Kowalczewska-Madura (2007). However, it was negatively correlated with Mollusca and total zooplankton (r = −0.67 and − 0.68, respectively). Also, total zooplankton was positively correlated with total Annelida and Nematoda counts (r = 0.91 and 0.522, respectively), while it was negatively correlated with total epiphytic microalgae. (r = −0.85). Cyanophyta (phytoplankton) was positively correlated with Rotifera, total epiphytic microalgae, Chlorophyta (epiphytic microalgae) and Arthropoda (r = 0.74, 0.85, 0.56, and 0.74, respectively).
These results indicated the cumulative effects of numerous environmental factors, organism requirements, and growth stages on behavior. It was therefore difficult to detect the main factors affecting distribution and diversity of aquatic organisms in the study area. These results were similar to findings reported by Abdel Gawad and Abdel-Aal (2018) Haroon et al. (2020), Tahoun et al. (2021) and Goher et al (2021b), where they recorded variable relations among epiphytes, macrophytes, phytoplankton, zooplankton, and macrobenthos at the Damietta branch and Rosetta branch of the Nile River, and at Lake Nasser.
3.4 Expected impacts of GRED
The GERD reservoir is intended to hold around 74 BCM of water at its full supply level (Ahmed and Elsanabary 2015). The design of GERD’s storage capacity was increased from an initial 11 BCM to 67 BCM (at 145 m height), then to 70 BCM, and finally to 74 BCM in 2012. Although the storage of water behind the GERD began in July 2020s and continued for the third time in 2022s with a storage capacity of 22 BCM of water (Ethiopian Monitor 2022), the effects on the Nile River and the High Dam Lake have not yet been determined. Until now, there has been no agreement on the expected effects of GERD on the downstream countries among scientists and riparian states. Some expect the positive effects of GERD for growth and prosperity, and others with the fact that GERD will be a curse in both Egypt and Sudan (Li et al. 2022).
Adverse impacts include the reduction of water level and storage volume in Lake Nasser, which may results on rapidly differences in the bathymetric chart of the lake (Abd Ellah and El-Geziry 2016). A reduction of energy generation of Aswan High Dam (AHD) especially through the filling period of GERD reservoir is, also, expected. The annual losses from AHD Lake is expected to increase by about 6% in case of AHD alone and the water salinity (TDS) will increase (Abdelhaleem and Helal 2015; El-Nashar and Elyamany 2018). Moreover, the river water temperature expected to be colder with about 0.5–1.5 °C and the velocity reduced by 5 to 42%. The changes in physicochemical characteristics of the water, frequently, are not favorable to the aquatic organisms in a given river, especially if there is an expected reduction in the water quality (Yacoub 2022). Additionally, The GERD blocks sediments that would naturally renew the downstream ecosystems (Ahmed and Elsanabary 2015), which may led to eroding in the river bed and banks which can undermine bridges, dams and other riverbank structures (Ahmed and Elsanabary 2015). Besides the concerns threats the river transportation, Nile tourism and fish farms. Concerning the agriculture sector, a reduction in the cultivated lands by 29.47% and 23.03% in Upper Egypt Delta is expected (CU 2013).
On the other side, the GERD can benefit Sudan and Egypt by removing up to 86% of silt and sedimentation, which increase the active storage capacity and operating life of the AHD. Also, the GERD will regulate the steady water flow throughout the year and it will avoid un-expected flooding to downstream countries. According to Wheeler et al. (2016) and Siddig et al. (2020), a positive impact is expected for Sudan due to the water flow regulation, where the hydropower generation in Sudan is expected to increase to around 20% and agriculture expanded and increasing the accumulated gross domestic product (GDP) by about US$ 27–29 billion until 2060. In all cases, Egypt will be affected by the lack of water coming through the Blue Nile, especially during the drought years, which may affect the water level in Lake Nasser and will have negative environmental impacts.
In addition to increasing the gap between the available water resources and the required needs, which will have many economic and social impacts. Therefore, Egypt needs to rationalize water consumption, use modern methods of agriculture, and increase the reuse of wastewater. This is in addition to coordination and cooperation with Ethiopia and Sudan to prevent a decrease in the water level in a lake to levels that may cause serious effects on Egypt's water security.
4 Conclusion
The findings of this study which held just before the start of filling the GERD confirm the good quality of Lake Nasser water. The majority of water constituents, except DO, showed a highly significant temporal difference (p < 0.001) between the two studied periods and between Dahmeit and Tushka Khors. The diversity, density and distribution of aquatic organisms (phytoplankton, zooplankton, macrophytes, epiphytic microalgae, and macrobenthos) were affected by environmental factors, organism behavior, growth stage, nutritional requirements, and allelopathic interactions between different organisms, indicating the cumulative effect of numerous variables which are all mainly affected by the flood cycle. The results in this study consider as a reference for the following studies on Lake Nasser khors after the complete filling of the GERD, where there are many expectations on the long-term downstream consequences of filling GERD. Although there are many possible scenarios for the effects of the Renaissance Dam on both Egypt and Sudan. However, all studies confirm the increase in the water deficit in Egypt as a result of the reduction of water discharge of the Blue Nile due to the water storage behind the Renaissance Dam, which may cause many environmental, economic, and social serious impacts. Consequently, the responsible authorities and the Egyptian government must develop urgent and long-term solutions to overcome any potential adverse effects.
Data availability
All data will be available from the corresponding author upon request.
References
Abd El-Karim MS (2014) Seasonality, biomasses and abundance of phytoplankton in the main khors of Lake Nasser. In: Environmental and biological monitoring of Lake Nasser for forcasting the impacts of the Ethiopian Renaissance Dam. National institute of oceanography and fisheries, First year survey pp 249–272
Abd El-Monem AM (1995) Spatial distribution of phytoplankton and primary productivity in Lake Nasser (PhD thesis) Univ. Collage for Girls (Egypt) Ain Shams University
Abd Ellah RG, El-Geziry T (2016) Bathymetric study of some khors in Lake Nasser, Egypt, Lakes, Reservoir and ponds. Roman J Limnol 10(2):139–158
Abdel Gawad SS, Abdel-Aal EI (2018) Impact of flood cycle on phytoplankton and macroinvertebrates associated with Myriophyllum spicatum in Lake Nasser Khors (Egypt). J Biol Sci 18(2):51–67. https://doi.org/10.3923/jbs.2018.51.67
Abdel-Aal EI (2021) Species composition and diversity of epiphytic microalgae on Myriophyllum spicatum on El-Ibrahimia Canal, Egypt. Afr J Aquat Sci 46(3):319–328. https://doi.org/10.2989/16085914.2021.1884523
Abdel-Hamid MI, Abdel-Aal EI, Azab YA (2014) Assessment of spatial quality improvement of a toxic industrial effluent based on physico-chemistry, changes in algal communities and algal bioassay. Afr J Aquat Sci 39(1):1–16. https://doi.org/10.2989/16085914.2013.870524
Abdel-Hamid MI, El-Amier YA, Abdel-Aal EI, El-Far GM (2017) Water Quality Assessment of El-Salam Canal (Egypt) based on physico-chemical characteristics in addition to hydrophytes and their epiphytic algae. Int J Ecol Dev Res 3(1):028–043
Abdelhaleem FS, Helal EY (2015) Impacts of grand Ethiopian renaissance dam on different water usages in upper Egypt. Brit J Appl Sci Technol 8(5):461–483
Adam MS, Hifney AF, Fawzy MA, Al-Badaani AA (2017) Seasonal biodiversity and ecological studies on the epiphytic microalgae communities in polluted and unpolluted aquatic ecosystem at Assiut, Egypt. Eur J Ecol 3:92–106
Ahmed AT, Elsanabary MH (2015) Environmental and hydrological impacts of grand Ethiopian renaissance dam on the Nile River. Int Water Technol J 5(4):260–271
Ali MM, Soltan MA (2006) Expansion of Myriophyllum spicatum (Eurasian water milfoil) into Lake Nasser, Egypt: invasive capacity and habitat stability. Aquat Bot 84:239–244. https://doi.org/10.1016/j.aquabot.2005.11.002
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, USA.WPCF, Washington, DC
Ayers R, Westcot D (1985) Water quality for agriculture. FAO Irrigation and Drainage Paper 29 (last updated 1994). http://www.fao.org/docrep/003/t0234e/t0234e00.HTM
Boulos L (2005) Flora of Egypt. Volume IV Monocotyledons (Alismataceae-Orchidaceae). Al-Hadara Publishing, Cairo, Egypt
Brinkhurst RO, Jamison BGM (1971) Aquatic Oligochaeta of the world. Tornto Press, Ontario, p 860
Brown DS (1980) Fresh water snails of Africa and their medical importance. Lond., Taylor &Francis Ltd
Burkepile DE, Hay ME (2006) Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology 87:3128–3139
Carvalho C, Hepp LU, Palma-Silva C, Albertoni EF (2015) Decomposition of macrophytes in a shallow subtropical lake. Limnological 53:1–9
CCME (2007) Canadian Council of Ministers of the Environment for the protection of aquatic life 2007. In: Canadian Environmental Quality Guidelines, 1999, Canadian Council of Ministers of the Environment, 1999, Winnipeg
Cleve-Euler A (1952) Die Diatomeen von Schweden und Finland. Band 1, 2, 3, 4 and 5. Almqvist and Wiksells Boktryckeri AB, Stockholm
Corlett H, Jones B (2007) Epiphyte communities on Thalassia testudinum from Grand Cayman, British West Indies: Their composition, structure, and contribution to lagoonal sediments. Sed Geol 194(3–4):245–262. https://doi.org/10.1016/j.sedgeo.2006.06.010
CU (2013) Implications of Bridging the Ethiopian Renaissance on Egypt, a conference organized by Nile Basin Group, Cairo University (CU), Cairo, 16 April 2013
Edmondson WT (1959) Fresh water biology, 2nd edn. Wiley, New York & London, p 1248
El Sayed SM, Hegab MH, Mola HRA, Ahmed NM, Goher ME (2020) An integrated water quality assessment of Damietta and Rosetta branches (Nile River, Egypt) using chemical and biological indices. Environ Monit Assess. https://doi.org/10.1007/s10661-020-8195-4
El-Enany HR (2009) Ecological studies on planktonic and epiphytic microinvertebrates in Lake Nasser, Egypt. Ph.D. Zool. Dept. Thesis Fac. Sci. Benha Univ
Elmorsi RR, Abou-El-Sherbini KS, Abdel-Hafiz Mostafa G, Hamed MA (2019) Distribution of essential heavy metals in the aquatic ecosystem of Lake Manzala, Egypt. Heliyon 5:e02276
El-Nashar WY, Elyamany AH (2018) Managing risks of the Grand Ethiopian Renaissance Dam on Egypt. Ain Shams Eng J 9:2383–2388. https://doi.org/10.1016/j.asej.2017.06.004
El-Serafy SS, Mageed AA, El-Enany HR (2009) Impact of flood water on distribution of zooplankton in the main Lake Nasser Khors, Egypt. J Egy Acad Soc Environ Dev 10(1):121–141
El-Shabrawy GM, Dumont HJ (2003) Spatial and seasonal variation of the zooplankton in the coastal zone and main khors of Lake Nasser, Egypt. Hydrobiologia 49:119–132
Ethiopian Monitor (2022) Third round filling of GERD Reservoir Complete, August 12, 2022. https://ethiopianmonitor.com/2022/08/12/third-round-filling-of-gerd-reservoir-complete/
EWQS (2007) Egyptian drinking water quality standards, Ministry of Health, Population Decision number 458
Goher ME, Ali MHH (2009) Monitoring of water quality characteristics and some heavy metals in water, sediment and macrophytes in Main Khors of Lake Nasser (Egypt). J Egypt Acad Soc Environ Dev 10(4):109–122
Goher ME, Hassan AM, Abdel-Moniem IA, Fahmy AH, El Sayed SM (2014) Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. Egypt J Aquat Res 40(3):225–233. https://doi.org/10.1016/j.ejar.2014.09.001
Goher ME, Abdo MH, Mangood AH, Hussein MM (2015) Water quality and potential health risk assessment for consumption of Oreochromis niloticus from El-Bahr El-Pharaony Drain, Egypt. Fresenius Environ Bull 24(11):3590–3602
Goher ME, El-Rouby WA, El-Dek SI, El Sayed SM, Noaemy SG (2018) Water quality assessment of Qarun Lake and heavy metals decontamination from its drains using nanocomposites. IOP Conf Ser Mater Sci Eng 464:012003. https://doi.org/10.1088/1757-899X/464/1/012003/pdf
Goher ME, Mahdy EM, Abdo MA, El-Dars FM, Korioum MA, Elsherif AAS (2019) Water quality status and pollution indices of Wadi El-Rayan lakes, El-Fayoum Egypt. Sustain Water Resour Manag 5:387–400. https://doi.org/10.1007/s40899-017-0162-z
Goher ME, Mangood AH, Mousa IE, Salem SG, Manar MH (2021b) Ecological risk assessment of heavy metal pollution in sediments of Nile River, Egypt. Environ Monit Assess 193:703. https://doi.org/10.1007/s10661-021-09459-
Goher ME, Napiórkowska-Krzebietke A, Aly W, El-Sayed SM, Tahoun UM, Fetouh MA, Hegab MH, Haroon AM, Sabae SA, Abdel-Aal EI, Nassif MG, Hussian AEM (2021a) Comprehensive insight into Lake Nasser environment: Water quality and biotic communities—A case study before operating the renaissance dam. Water 13(16):2195. https://doi.org/10.3390/w13162195
Goldman JC, McCarthy JJ, Peavey DG (1979) Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 279:210–215
Gołdyn R, Kowalczewska-Madura K (2007) Interactions between phytoplankton and zooplankton in the hypertrophic Swarzędzkie Lake in western Poland. J Plankton Res 30(1):33–42
Habashi F (1993) A textbook of hydrometallurgy. Métallurgie Extractive Québec, Quebec City
Haroon AM, Abd Ellah R (2021) Variability response of aquatic macrophytes in inland lakes: a case study of Lake Nasser. Egypt J Aquat Res. https://doi.org/10.1016/j.ejar.2021.07.004
Haroon AM, Abdel-Aal EI (2016) Chemical composition and in vitro anti-algal activity of Potamogeton crispus and Myriophyllum spicatum extracts. Egypt J Aquat Res 42:393–404
Haroon AM, Tahoun UM, Sabae SA, Hamza WT (2020) Biological characterization of water in Damietta branch of the Nile River, Egypt. Pak J Biol Sci 23:861–882. https://doi.org/10.3923/pjbs.2020.861.882
Harris GP (1986) Phytoplankton ecology: structure, function, and fluctuation. Chapman and Hall, London
Hassan FM, Salman JM, Alkam FA, Jawad HJ (2014) Ecological observations on Epipelic Algae in Euphrates River at Hindiya and Manathira, Iraq. Int J Adv Res 2(4):1183–1194
Hassan G, Goher ME, Shaheen ME, Taie SA (2021) Hybrid predictive model for water quality monitoring based on sentinel-2A L1C data. IEEE Access 9:65730–65749. https://doi.org/10.1109/ACCESS.2021.3075849
Hassouna EM, Goher ME, El-Sayed SM, Hassan RA (2019) Integrated approach quality indices and health risk assessment for Bahr Yusuf Canal water, Fayoum, Egypt. Oceanol Hydrobiol Stud 48(4): 337–354. 10.https://doi.org/10.2478/ohs-2019-0031
Hussian AM, Haroon AM (2019) Sensitivity of submerged aquatic macrophytes and their epiphytic microalgae to the different environmental variables in River Nile, Egypt. Int J Ecol Environ Sci 45(1):107–122
Hussian AM, Napiórkowska-Krzebietke A, Toufeek MEF, Abd El-Monem AM, Morsi HH (2015) Phytoplankton response to changes of physicochemical variables in Lake Nasser, Egypt. J Elem 20(4):855–871. https://doi.org/10.5601/jelem.2015.20.1.895
Imam N, El-Sayed SM, Goher ME (2020) Risk assessments and spatial distributions of natural radioactivity and heavy metals in Nasser Lake, Egypt. Environ Sci Pollut Res 27:25475–25493. https://doi.org/10.1007/s11356-020-08918-7
Iskaros IA, El-Dardir M (2010) Factors affecting the distribution and abundance of bottom fauna in Lake Nasser, Egypt. Nat Sci 8(7):95–108
Jeffrey SW, Vesk M (1997) Introduction to marine phytoplankton and their pigment signatures. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO, Paris, pp 19–36
Kawecka B, Eloranta PV (1994) The outline of algae ecology in freshwater and terrestrial environments. PWN Scientific Publishers, Warsaw, pp 1–252 (in Polish)
Khalifa N, Bendary RE (2016) Composition and biodiversity of zooplankton and macrobenthic populations in El-Rayah El Menoufy. Int J Appl Environ Sci 11(2):683–700
Khalifa N, El-Damhogy KA, Fishar MR, Nasef AM, Hegab MH (2015) Vertical distribution of zooplankton in Lake Nasser, Egyptian. J Aquat Res 41:177–185
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33(6):1441–1450. https://doi.org/10.2307/2404783
Komárek J, Zapomělová E (2007) Planktic morphospecies of the cyanobacterial genus Anabaena= subg. Dolichospermum–1. Part: coiled types. Fottea 7(1):1–31
Körner SH (2006) Nee Allelopathic inhibition of epiphytes by submerged macrophytes. Aquat Bot 85:252–256
Kupferberg S (2003) Facilitation of periphyton production by tadpolegrazing: functional differences between species. Freshw Biol 37:427–439
Latif AFA (1984) Lake Nasser-the new man-made lake in Egypt (with references to Lake Nubia). In: Ecosystem of the world lakes and reservoirs, p 23
Leterme SC, Seuront L, Edwards M (2006) Differential contri-bution of diatoms and dinoflagellates to phytoplankton biomass in the NE Atlantic Ocean and the North Sea. Mar Ecol-Prog Ser 312:57–65
Li P, He Z, Cai J, Zhang J, Belete M, Deng J, Wang S (2022) Identify the impacts of the grand Ethiopian Renaissance Dam on watershed sediment and water yields dynamics. Sustainability 14(13):7590. https://doi.org/10.3390/su14137590
Madden CP (2010) Key to genera of larvae of Australian Chironomidae (Diptera). Museum Victoria Sci Rep 12:1–31
Nassif MG (2021) Biodiversity and distribution of macrobenthic invertebrate community in Lake Nasser, Egypt. Egypt J Aquat Biol Fisheries 25(5):769–786. https://doi.org/10.21608/ejabf.2021.206680
Othman AA, Haroon AM (2020) Association between the distributions and chemical composition of aquatic macrophytes and bacterial community structure in some irrigation canals (Rayahs) of the Nile River, Egypt. Egypt J Aquat Biol Fish 24(5): 639–660
Parmar TK, Rawtani D, Agrawal YK (2016) Bioindicators: The natural indicator of environmental pollution. Front Life Sci 9:110–118
Pascal PY, Fleeger JW (2013) Diverse dietary responses by saltmarsh consumers to chronic nutrient enrichment. Estuaries Coasts 36:1115–1124
Pennak RW (1978) Fresh-water invertebrates of the United States, 2nd edn. Wiley, New York
Pouličkowá A, Duchosłav M, Doculil M (2004) Littoral diatom assemblages as bioindicators of lake trophic status: a case study from perialpine lakes in Austria. Eur J Phycol 39:143–152
Prescott GW (1978) How to know the fresh water algae. Brown Company publishers, Dubuque, p 12
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Rizk R, Juzsakova T, Cretescu I, Rawash N, Sebestyén V, Le Phuoc C, Kovács Z, Domokos E, Rédey A, Shafik H (2020) Environmental assessment of physical-chemical features of Lake Nasser, Egypt. Environ Sci Pollut Res 27:20136–20148. https://doi.org/10.1007/s11356-020-08366-3
Samaan AA, Gaber MA (1976) Report on plankton investigation in Lake Nasser during March and August, 1976. In: Latif AFA (ed) Reports on surveys to Lake Nasser & River Nile Project. Acad. Sci. Res. & Tech., Cairo, Egypt
Sensorex (2021) The importance of dissolved oxygen in water and water systems. https://www.lenntech.com/why_the_oxygen_dissolved_is_important.htm., last visited October, 16, 2021
Shalloof KA, Alaa M, El-Far AM, Walid Aly W (2020) Feeding habits and trophic levels of cichlid species in tropical reservoir, Lake Nasser, Egypt. Egypt J Aquat Res 46(2):159–165. https://doi.org/10.1016/j.ejar.2020.04.001
Sharshar KM, Haroon AM (2009) Comparative investigations on some biological and biochemical aspects in freshwater crayfish (Procambarus clarkii) fed on Eichhornia crassipes, Echinochloa stagnina L. and Polygonum tomentosum. Am Eurasian J Agric Environ Sci 5:579–589
Siddig K , Basheer M , Abdelhamid A (2020) Implications of the initial filling of the Grand Ethiopian Renaissance Dam for the Egyptian Economy. GTAP 2020 conference paper. https://www.gtap.agecon.purdue.edu/resources/download/9781.pdf
Slavevska-Stamenković V, Smiljkov S, Prelić D, Paunović M, Atanacković A, Rimcheska B (2010) Structural characteristic of benthic macroinvertebrate in the Mantovo Reservoir (South-East Part of the R. Macedonia). BALWOIS 2010-Ohrid, Republic of Macedonia
Sleem S, Hassan MM (2010) Impact of pollution on invertebrates biodiversity in the River Nile Associated with Dahab and El-Warrak Islands, Egypt. Int J Enviro Sci Eng 1:15–25
Taha OE, Mageed AA (2002) Spatial distribution and relationship between phytoplankton and zooplankton in Lake Nasser (Egypt) after the flood season. Egypt J Aquat Biol Fish 6(4):265–281
Tahoun U, Haroon AM, Elsebaie H, Sabae S, Hamza WT, Abdel-molla H (2021) Qualitative and quantitative variability of Flora and Fauna Talong Rosetta Branch of Nile River, Egypt. Egypt J Aquat Biol Fish 6:66
Talab AS, Goher ME, Ghannam HE, Abdo MH (2016) Seasonal changes effect on the proximate composition, physicochemical quality and heavy metal concentrations in Nile Tilapia from the Nile Rayahs. Egypt J Aquat Res 42(1):23–31. https://doi.org/10.1016/j.ejar.2016.01.003
Taylor JC, Harding WR, Archibald CGM (2007) An illustrated guide to some common diatom species from South Africa. (WRC Report TT 282/07). 178 plates
Ter Braak CJF, Smilauer P (1998) Canoco reference manual and user guide to Canoco for Windows, software for Canonical community ordination (version 4) centre for Biometry Waginengen
Tiffany MA (2011) Epizoic and epiphytic diatoms. In: Seckbach J, Kociolek JP (eds) The diatom World. Springer, pp 195–211
Trombetta T, Vidussi F, Mas S, Parin D, Simier M, Mostajir B (2019) Water temperature drives phytoplankton blooms in coastal waters. PLoS ONE 14(1):e0214933
UNP (1997) United Nations Publication, Glossary of Environment Statistics, Studies in Methods, Series F, No. 67, United Nations, New York, 1997. https://unstats.un.org/unsd/publication/seriesf/seriesf_67e.pdf
USEPA (2018) United States Environmental Protection Agency, Regional Screening Levels (RSLs)—Generic Tables, Summary Table, Nov. 2018. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables
USEPA (2021) United States Environmental Protection Agency, National Recommended Water Quality Criteria—Aquatic Life Criteria Table. https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table. Last visited October. 22, 2021
USGS (2021) United States Geological Survey, Water Quality Topics, pH and water. https://www.usgs.gov/special-topic/water-science-school/science/ph-and-water?qt-science_center_objects=0#qt-science_center_objects. Last visited October, 19, 2021
Verheye HM, Dumont HJ (1984) The calanoid copepods of the Nile system. Hydrobiology 110:191–212
Wahab HM, Iskaros IA, Fishar MR, Mohamed NE (2018) Ecological studies on macrobenthic invertebrates in four northern Khors of Lake Nasser (Egypt). Egypt J Aquat Biol 22(3):111–124
Wehr JD, Sheath RG (2003) Freshwater habitats of algae. Freshwater Algae of North America. Academic Press, San Diego, p 308
Wheeler KG, Basheer M, Mekonnen Z, Eltoum S, Mersha A, Abdo G, Zagona E, Hall J, Dadson S (2016) Cooperative filling approaches for the Grand Ethiopian Renaissance Dam. Water Int 8060:1–24. https://doi.org/10.1080/02508060.2016.1177698
WHO (2017) World Health Organization, guidelines for drinking water quality. The fourth edition incorporating the first addendum. https://www.who.int/water_sanitation_health/water-quality/guidelines/en/
Wirth WW, Stone A (1956) Aquatic Diptera, p. 372–482. In: Usinger RL (ed) Aquatic insects of California. University of California Press, Berkeley, p 508
Wissa JB (2002) Ecological studies on benthic invertebrates of Nile River at north Cairo region. M. Sc. Thesis, Fac. Sci., Ain Shams Univ
Yacoub AR (2022) The right to the world’s longest river: reopening the vexing case of the Nile River. William & Mary Environ Law Policy Rev 46(2):421–475
Yuan LL, Pollard AI (2018) Changes in the relationship between zooplankton and phytoplankton biomasses across a eutrophication gradient. Limnol Oceanogr 63(6):2493–2507
Zaher SS, Aly W (2021) Impact of flood regime on phytoplankton communities in the large African reservoir, Lake Nasser, Egypt. Afr J Aquat Sci 46(3):340–352
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Field study and sampling, Mohamed E. Goher; Sample processing and Analysis, All authors; writing of the first draft and final text, all authors; review, editing and approving of the final text, all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Consent to participate
All authors voluntarily agree to participate in this research study.
Consent to publish
All authors voluntarily approved the publication of this research study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Aal, E.I., Haroon, A.M., Ibrahim, S.M. et al. Ecological status of Lake Nasser Khors, Egypt, before operating the Grand Ethiopian Renaissance Dam. Stoch Environ Res Risk Assess 37, 1229–1245 (2023). https://doi.org/10.1007/s00477-022-02329-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00477-022-02329-5