Abstract
Leave’s vein xylem and stomata hydraulic traits should be critically linked to modulating plant responses to drought in leaves of desert species, influencing traits at the whole-plant level and promoting adaptation. We tested for coordination among leaf vein xylem anatomical traits across different hierarchical orders (hydraulic vessel diameter, vein area, free veins per area, areolas per area, total free veins number, total areola number) and stomatal traits (stomatal size, stomatal density, stomatal index, leaf total stomatal area, leaf total stomatal number) as well as their relationship with the leaf area in ten tree species from the Sonoran Desert scrub, Mexico. Moreover, these traits were correlated with other hydraulic and functional traits associated with resource use strategies (hydroscape area, stem-specific density, leaf mass per area, and leaf phenology). Leaf total stomatal area and number were positively associated with vessel diameters at the midrib and with leaf total free terminal veins and areola number, indicating coordination between water transpiration and transport. Also, interspecific differences fit species along a physiological resource use spectrum, following an exploitative vs. conservative physiological axis. Accordingly, species with lower leaf longevity (LL) and foliage duration at the canopy (FD) displayed higher values of stomatal indices and free vein densities, higher stomata density; and anatomical traits related to higher hydraulic conductance and gas exchange in comparison to species with higher LL and FD. Therefore, in this community, species have been selected to take advantage of different temporal hydrological niches to enhance survival under unpredictable and highly seasonal water availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants from arid regions face low water availability as their most important selective pressure (González-Medrano 2012). Therefore, species adaptation to cope with this type of stress has probably modified their anatomy and physiology (Dimmitt 2015), ranging from drought-avoidant species (deciduous woody species and ephemeral herbaceous species with fast growth) to drought-resistant species (long-lived evergreen species with slow growth) (Dimmitt 2015, Lambers y Oliveira 2019). Leaves are the prime target for selection to control water loss and transportation, because they are responsible for gas exchange through stomata, which is at the same time the source of transpiration and the main resistance to water conductivity at the whole plant level (Sack and Tyree 2005; Sack and Holbrook 2006). As desert ecosystems will be the model for the reaction of several other systems to the incumbent climatic changes of the XXI century (IPBES 2018), it is critical to quantify the hydraulic characteristics of leaves from desert plants to understand and predict species’ ecophysiological responses under intensifying drought regimes.
The structure and function of the leaves involve an inherent anatomical and physiological coordination between the traits responsible for water delivery (xylem traits) and those related to water loss and CO2 uptake through the stomata (Brodribb et al. 2017). The stoma-xylem physiological link is subject to a trade-off between enhancing water transpiration and acquiring CO2 versus restricting transpiration and reducing the probability of leaf hydraulic pathways collapse but decreasing CO2 uptake (Brodribb et al. 2017; Li et al. 2018). Therefore, the number and size of xylem and stomata, in addition to leaf area of each leaf show coordinated development throughout the leaf ontogeny to optimize leaf xylem water transport and stomatal transpiration as leaves grow by passive epidermal cell expansion (Carins Murphy et al. 2016; Brodribb et al. 2017). Thus, it is no surprise that leaf xylem (veins) and stomata traits are also associated with other critical leaf water transport and carbon assimilation traits. For water to reach the evaporation surfaces at the mesophyll, at the stomatal chambers, water should be conducted from basal primary veins at the petiole to the minor veins, thus, vessel tapering should occur to maximize the coverage of the leaf surface (Coomes et al. 2008; Sack et al. 2012; Zhong et al. 2019), in turn, vessel widening from leaves to stem and roots should occur to reduce water resistance from leaves to roots of trees of different heights and higher transpiration surface (Olson et al. 2021). This vessel tapering and widening is expected to be strongly related to the leaf area, because an important plant strategy is maintaining constant water supply and demand per leaf area (Echeverria et al. 2019, Zhong et al. 2020). In addition, the minor vein density also influences the leaf's hydraulic conductance. Minor vein density also shows correlations to stomatal density, which, in turn, directly influences the maximum stomatal conductance rate (Lawson and Matthews 2020; Sack et al. 2013). Finally, as the stomatal conductance of the leaf is strongly correlated to the photosynthetic rate per area, we might expect anatomical leaf xylem and stomatal traits to be strongly related to the plant functional traits encompassed in the plant economic spectrum (PES, Reich 2014; Volaire 2018). Furthermore, we might expect these anatomical traits to be related to the iso-anisohydric continuum (Sack et al. 2013; Brodribb et al. 2017; Fu et al. 2019; Chen et al. 2021; Gonzalez-Rebeles et al. 2021).
The plant economic spectrum framework considers that there is a trade-off in resource acquisition (Reich 2014). On one extreme, there are plants that take advantage of the favorable rainy season and have a fast acquisition of resources. These plants are deciduous or annual plants with cheap leaves, low leaf mass per area (LMA), low leaf longevity (LL) or foliage duration (FD), and low stem density (SSD, Wright et al. 2004, Mendez-Alonzo et al. 2012, Gonzalez-Rebeles et al. 2021), which is related to a higher capacity to move water through the xylem (Chave et al. 2009). At the other end of the spectrum, some species have a slow acquisition of resources throughout the year. These plants are evergreen with expensive leaves with high LL and FD (Wright et al. 2004, Mendez-Alonzo et al. 2012, Gonzalez-Rebeles et al. 2021). In addition, they can present a higher stem density (Mendez-Alonzo et al. 2012, Gonzalez-Rebeles et al. 2021) which is associated with protection against xylem failure during water stress (Chave et al. 2009).
Plant species can also be classified in the iso-anisohydric continuum depending on their stomatal sensitivity and fluctuations in water potential in response to the decrease of soil water availability. When soil water availability is reduced, the isohydric species show a faster stomatal closure without substantial changes in leaf water potential. In contrast, the anisohydric species maintain their stomata open, supporting higher fluctuations in their leaf water potentials (Tardieu and Simonneau 1998; Meinzer et al. 2016). In turn, the iso-anisohydric continuum is associated with the PES (Fu et al. 2019; Fu and Meinzer 2019; Chen et al. 2021, González-Rebeles et al. 2021), with anisohydric species being resource conservative and resistant to cavitation, and isohydric species being resource exploitative but vulnerable to cavitation (González-Rebeles et al. 2021). This study aims to find the links between leaf xylem vessel widening and stomatal traits and their relationship with phenology and functional traits that define the PES and iso/anisohydric continuum in trees from the Sonoran Desert.
The Sonoran Desert is one of the most diverse deserts in North America (Shreve 1951). Previous studies have shown the high functional diversity of tree species related to phenology and their responses to drought (González-Rebeles 2021, 2023). Canopy foliage duration, gas exchange, hydraulic, and other functional traits allowed classifying the species considering the iso-anisohydric and fast-slow resource use frames. As structure and function should show strong evolutionary couplings, we hypothesize species to have interspecific differences in their anatomical traits related to water demand and water supply. In addition, we hypothesize that the scaling leaf vein and stomatal traits will be related to leaf area, reflecting a way to maintain a constant leaf hydraulic and photosynthetic productivity per leaf area. We also expect a correlation between anatomical xylem and stomatal traits reflecting water supply and demand coordination. Furthermore, we hypothesize that these species would diverge in their leaf vein and stomatal traits in concert with their water and carbon resource use strategies; with anatomical traits being coordinated with traits associated with the iso-anisohydric continuum and the PES. These expectations were explored by testing the associations of several stomatal traits (stomatal size, stomatal density, stomatal index, leaf total stomatal area, leaf total stomatal number) with leaf vein xylem traits of different orders (hydraulic vessel diameter, vein area, free veins per area, areolas per area, total free veins number and total areola number) and in their relation with traits of the iso-anisohydric continuum (hydroscape area) and PES (stem-specific density, leaf mass per area, leaf phenology) across ten species of trees of the Sonoran Desert Scrub vegetation close to Hermosillo, Sonora, Mexico.
Materials and methods
Study sites
This study was performed in two sites covered by desert scrub vegetation: the archeological reserve La Pintada (28º 35′ 18’’ N 110º 57′51’’W; elevation 269 m a.s.l.), and the Centro Ecológico del Estado de Sonora (CEES, 29º 01′41’’N 110º 57′09’’ W; elevation 245 m a.s.l.), both within the southern part of the Sonoran Desert (Shreve and Wiggins 1964). Within the study area, the short-wet season occurs from July to October, and the long dry season from November to June. Precipitation occurs mainly due to summer monsoon rains (ca. 80% annual precipitation) and secondarily due to low-intensity precipitation events during winter. However, the intensity of monsoon rains varied greatly throughout the years, and the intervals between rain events may be highly unpredictable (Ezcurra and Rodrigues 1986). Summer maximum temperatures are commonly above 40 °C; winter minimum temperatures may be lower than 10 °C, and frost is absent, allowing the persistence of high plant diversity, including elements from the Neotropical and Nearctic biogeographical realms (Brito-Castillo et al. 2010). In La Pintada, the mean annual precipitation for the last 9 years (2014–2022) was 342 mm, and the average maximum temperature was 45.7 °C (Ortiz Meteorological station, CESAVE-SIAFESON 2023). In CEES, the mean annual precipitation for the same period was 386.5 mm, and the average maximum temperature was 45.2 °C (La Tracolita Meteorological station, CESAVE-SIAFESON 2023).
Species selection
We selected ten representative tree species of the Sonoran Desert Vegetation based on previous knowledge of their foliar phenology (Turner et al. 1995, Table 1). In previous studies, we characterized their phenology, several hydraulic, morphological, and physiological characteristics of leaves and stems for adult trees, and their foliar dynamics were monitored (González-Rebeles et al. 2021). Also, the following anatomical characteristics were measured: the hydraulically weighted mean vessel diameter of the stem, midrib, and petiole, as well as the stomatal index of the leaf abaxial surface (González-Rebeles et al. 2021). In this study, we deepen the anatomical analysis by quantifying 20 anatomical leaf characteristics of the same trees related to leaf hydraulics and gas exchange. We also correlated these characteristics with their respective plant phenological and physiological traits (González-Rebeles et al. 2021).
The studied species presented either simple or compound leaves (Table 1), and we considered the leaflet and the petiolule of the compound leaf species as an anatomical and functional equivalent to the leaf and petiole of the simple leaf species for all the morphological and anatomical analyses (Niinemets 2006). The petiolule will be referred to as the petiole and the leaflets as leaves in the rest of the document.
To evaluate the physiological and anatomical relationships and differences among species, 27 traits were considered (Table 2). These included 20 newly quantified anatomical traits in determining the structural coordination of stomata and leaf vein xylem. In addition, we included seven previously measured traits in the same species and individuals, including hydroscape areas, leaf phenology traits (leaf longevity, LL, and canopy foliage duration, FD), and morphological traits (leaf mass per area, LMA, and stem-specific density, SSD). Methods and values for the hydrological, phenological, and morphological traits were extracted from Gonzalez-Rebeles et al. (2021).
Anatomical traits of branches
Branches from 0.68 cm to 1.01 cm in diameter, being 4–5 years old (five scars from the apex), were collected from five individuals per species in September 2017. A portion of the stems collected was fixed in a solution of formaldehyde-acetic acid–ethanol (FAA, Ruzin 1999) for 24 h and then washed and stored in a glycerol-ethanol–water (1:1:1) solution until sectioning. Afterward, the sections were cut in a sliding microtome (18–20 µm thick), stained with safranin-fast green, and mounted in synthetic resin (Terrazas et al. 2011).
Vessel frequency per square mm (F) was counted in 10 × magnification in a field of a size area of 0.747 mm2, and the diameter of 50 vessels per individual (d) was measured at a 40 × magnification using an image analyzer (Image-Pro, Media-Cybernetics, Rockville USA). In the transverse stem sections, vessel diameter (d) was calculated to obtain the hydraulically weighted mean vessel diameter of the stem (dhS, Coomes et al. 2008, González-Rebeles et al. 2021):
Leaf anatomical traits
Leaves of five individuals per species were collected at the end of the growing season (September 2017) for leaf anatomical determinations. Leaf segments were fixated in FAA (Ruzin 1999) for 24 h and then introduced in 60% ethanol. Then, the segments were dehydrated in a tissue processor (TP1020, Leica Wetzlar, DE) and embedded in paraffin following Martínez-Cabrera et al. (2009). Transverse and paradermal sections of petioles and laminas 12–14 µm thick laminas were sectioned with a rotary microtome, stained with safranin-fast green, and mounted in synthetic resin (Terrazas et al. 2011). As in the case of branches, the hydraulically weighted mean vessel diameter (dhP, from Coomes et al. 2008) was obtained for the base of the petioles using the same set of methods.
Leaf anatomical traits transverse sections
In the transverse sections of petioles and lamina, vessel diameter (d) was calculated to obtain the hydraulically weighted mean vessel diameter of the terminal vessels (dhT), the vessels of the secondary veins (dh2), the midrib (dh1) and the petiole (dhP), by also calculating the relative conductance following Coomes et al. (2008).
Vascular bundle area was calculated by drawing a circumference in the vascular bundles (only the area occupied by the xylem without considering phloem or fibers). In some species, there was more than one vascular bundle in midvein and petiole. Therefore, we summed all the vascular bundles to obtain the total vascular bundle area. We obtained the vascular bundle area of the terminal vein (VAT), secondary vein (VA2), midrib vein (VA1), and petiole (VAP).
Leaf anatomical traits paradermal sections
In the paradermal sections, for each individual, we counted the number of terminal veins and areolas at a 10 × magnification in five fields of a size area of 0.747 mm2 each. The number of free terminal veins and areolas was divided by the field size of 0.747 mm2 to obtain the number of free terminal veins per area (TV/A) and the number of areolas per area (A/A). The leaf total free terminal vein number (LTVN) was calculated by multiplying the leaf area by TV/A. The leaf total areola number (LTAN) was calculated by multiplying the leaf area by A/A.
For each individual, in the paradermal sections of the abaxial and adaxial surface, we counted the number of stomata at a 40 × magnification in five fields of a size area of 0.046 mm2 each. For the hypostomatic species (Bufa, Bula, Jama, Lydi, Pami, and Sasa, abbreviations in Table 1), we obtained the stomatal density (StoD) by dividing the number of stomata observed in the abaxial surface by the field area. For the amphistomatic species (Foma, Guco, Jaco, Olte), we obtained the abaxial stomatal density (StoDab) and the adaxial stomatal density (StoDad) by dividing the number of stomata observed on each surface by the field area, calculating the average stomatal density (StoD) the following way (El-Sharkawy et al. 1985, Melgarejo et al. 2010):
Epidermal cell density (ED) was also quantified per square millimeter. For the amphistomatic species, the same method of El-Sharkawy et al. (1985), as described above, was used to obtain an average epidermal cell density. The Salisbury stomatal Index (StoI, Salisbury 1927; Navea et al. 2002) was estimated as:
Stomatal cell area (SCA) was calculated by measuring the maximum width (W) and maximum length (L) of 10 guard cells of 10 closed stomata in each leaf in a 40 × magnification and, using the formula of Zhong et al. (2020):
Leaf total stomatal area (LSN) was obtained by multiplying StoD by LA and SCA. Leaf total stomatal number (LSN) was calculated by multiplying StoD by LA (Zhong et al. 2020).
Data analysis
Interspecific differences between measured traits, but controlling the lack of independence of species due to common evolutionary history (Garland et al. 1993), were analyzed using One-way phylogenetic ANOVA (phyANOVA), a procedure that ponders the degrees of freedom and the significance value of phylogenetically close species using permutation and bootstrap simulations using the phytools package in R (Adams and Collyer 2018; Table 2).
To understand the relation between the 27 traits we calculated a multiple Pearson’s correlation analysis in JASP (JASP team, 2023, Table S1). To determine the leaf scaling of the hydraulically weighted mean vessel diameter (dh) and the vessel area (VA) of the petiole, midrib, secondary vein, and midrib, for each species we fitted straight lines to a ln-ln transformed data by using standardized major axis regression with the SMATR package in R (R Development Core Team, 2020), considering alpha (α), the slope, as an indicator of the scaling relationship across vein hierarchical orders. We calculated the regressions of the hydraulically weighted mean vessel diameter (dh) and the Ln-transformed vessel area (VA) of the petiole, midrib, secondary vein, and midrib with the Ln(x + 1)-transformed values of leaf area. Also, we calculated the relation between stomatal density (StoD), stomatal index (StoI), and stomatal area (StoA) with the Ln(x + 1)-transformed values of leaf area. To summarize the set of associations among xylem, stomata, morphological and phenological traits, we conducted a principal component analysis using a matrix of the mean trait values of HA, FD, LL, VA1, dh1, VAP, dhP, StoI, TV/A, LA, SSD, LMA, VAT for the ten species using the FactomineR package in R (R Development Core Team, 2020). Graphs were designed and edited in GraphPad Prism (version 8.3.0 for Mac OS, GraphPad Software, San Diego, California USA).
Results
Interspecific differences between traits
With the phylogenetic ANOVA, we found significant differences among species in phenology, morphology, and anatomy: for leaf longevity (LL), canopy foliage duration (FD), leaf mass per area (LMA), leaf area (LA), hydraulically weighted mean vessel diameter of the petiole (dhP), number of free terminal veins per area (TV/A), leaf total free terminal vein number (LTVN) (Table 2).
Leaf vessel scaling and its relation to leaf area
At the leaf level, species presented different relations in the slope or the scaling (α) of the xylem vessel diameter to the vascular bundle area (dh ~ VA; Table 3, Fig. S1). Species presented an average α of 0.10, with a maximum of 0.18 in Lydi and a minimum of − 0.04 in Pami. While most species had a reduction in vessel diameter from the petiole to the terminal veins, Boma, Olte, and Pami did not show evidence of tapering. In some cases, terminal vein diameters were even larger than secondary vein diameters, which affected α. For example, in Pami, the xylem vessel diameters of the terminal veins were larger than those of the secondary veins, and that causes it to have a negative slope, and therefore a negative scaling relationship. The vessel scaling (α) was significantly correlated with the petiole vessel diameter (dhP, rp = 0.68, p < 0.05). Species with larger differences between vein orders (higher α) presented larger dhP than species with lower α (Table S1, Supplementary Material).
Leaf area was correlated with most of the leaf xylem anatomical traits measured, except for the diameters of terminal veins (dhT) and vascular bundle area of terminal veins (VAT) (Table S1). We found significant positive correlations between the hydraulically weighted mean vessel of the petiole (dhP, R2 = 0.48), of the midrib (dh1, R2 = 0.52), and the secondary vein (dh2, R2 = 0.68) to the ln(x + 1)-transformed leaf area (Fig. 1 A, B, C). In addition, the ln-transformations of the vascular bundle area of the petiole (VAP, R2 = 0.68), of the midrib (VA1, R2 = 0.53), and the secondary vein (VA2, R2 = 0.59) were also significantly and positively correlated to the ln(x + 1)-transformed leaf area (Fig. 1 D, E, F). In contrast, there were no significant associations between the stomatal density (StoD, R2 = 0.12), stomatal index (StoI, R2 = 0.10), and stomatal cell area (StoA, R2 = 0.31), or α (R2 = 0.17) with the ln(x + 1)-transformed leaf area.
Linear regressions between leaf area (ln (LA + 1)) and A) hydraulically weighted mean vessel diameter of the petiole (dhP), B) midrib vein (dh1), and C) secondary vein (dh2). In addition, linear regression between the ln-transformed leaf area (ln (LA + 1)) and D) Ln-transformed vascular bundle area of the petiole (ln VAP), E) midrib vein (ln VA1), and F) secondary vein (ln VA2) for ten tree species of the Sonoran Desert Scrub
Relationship between leaf xylem and stomatal traits
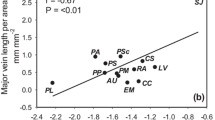
There were significant correlations with stomatal and xylem traits (Fig. 2, Table S1). Species with higher leaf stomatal number (LSN) presented higher vascular bundle area of the petiole (VAP, rp = 0.83, p < 0.01), midrib (VA1, rp = 0.81, p < 0.01) and secondary vein (VA2, rp = 0.67, p < 0.05), and higher leaf total vein number (LTVN, rp = 0.64, p < 0.05). Species with higher leaf stomatal area (LSA) presented higher hydraulic vessel diameter of the petiole (dhP, rp = 0.65, p < 0.05), midrib (dh1, rp = 0.64, p < 0.05) and secondary vein (dh2, rp = 0.73, p < 0.05). Species with higher LSA also presented higher VAP (rp = 0.95, p < 0.001), VA1 (rp = 0.69, p < 0.05), VA2 (rp = 0.85, p < 0.01), LTVN (rp = 0.97, p < 0.001) and leaf total areola number (LTAN, rp = 0.94, p < 0.001). Species with higher stomatal index (StoI) presented a higher number of free terminal veins per area (TV/A, rp = 0.82, p < 0.01), a higher number of areolas per area (A/A, rp = 0.75, p < 0.01) and lower hydraulic vessel diameter of the terminal vein (dhT, rp = − 0.69, p < 0.05). Species with higher stomatal cell area (StoA) presented higher terminal vein vascular bundle area (VAT, rp = 0.72, p < 0.05) and vessel diameter (dhT, rp = 0.70, p < 0.05), (Table S1).
Heat map for Pearson’s significant correlations between xylem and stomata traits of 10 tree species of the Sonoran Desert shrub. Different shading represents different P-values (< 0.001 darker, < 0.01 medium, < 0.05 lighter, non-significant, or n.s. white). Traits abbreviations as indicated in Table 2
General Xylem-Stomata coordination and their relationship to traits related to the iso-anisohydric continuum and the plant economic spectrum.
With Pearson’s correlation analysis, we found that the more anisohydric species (higher hydroscape area, HA) were correlated with lower vessel diameter in the petiole (dhP, rp = − 0.660, p < 0.05), midrib (dh1, rp = − 0.743, p < 0.05) and secondary vein (dh2, rp = − 0.695, p < 0.05) in comparison with the less anisohydric species (Table S1, Fig. 3A). In addition, species with higher stem density were related to a lower dhP (rp = − 0.722, p < 0.05), dh1 (rp = − 0.818, p < 0.01), and dh2 (rp = − 0.816, p < 0.01), (Fig. 3B). Also, species with higher leaf mass per area (LMA) presented higher VAT (rp = 0.779, p < 0.01), (Fig. 4).
With the PCA analysis (Fig. 5), we found that the more isohydric species (lower HA) presented higher xylem vessel diameter in the petiole and the midrib, a higher petiole vascular bundle area (VAP) but lower stem-specific density and leaf mass per area than the more anisohydric species. Also, species with higher leaf longevity and leaf foliage duration presented a higher terminal vessel bundle area but a lower stomatal index and free veins per area than those with lower leaf longevity.
Species (A) and variables (B) plots derived from the two principal axes obtained from a principal component analysis summarizing 13 quantitative traits for ten species of the Sonoran Desert Scrub, Sonora, México. (A) Dispersion of species across the morphophysiological space, abbreviations follow Table 1. Symbols: Closed triangles = evergreen species with simple leaves, closed circles = evergreen species with compound leaves, Open triangles = deciduous species with simple leaves, open circles = deciduous species with compound leaves. The species in the green circle are evergreen, and the species in the orange circle are deciduous. (B) Trait correlative patterns and traits associated with PC1 are present in solid black lines, traits correlated with PC2 are shown with dashed lines, traits correlated with both PC1 and PC2 are shown in gray lines (Table S2). Trait abbreviations as in Table 2
Discussion
We found interspecific differences in anatomical characteristics in leaf xylem traits, as well as significant associations between anatomical, hydraulic traits and phenological, physiological, and morphological traits across species. In addition, leaf area was strongly related to traits associated with water transport in the leaf, indicating a strategy to maintain the water supply constant independently of the leaf size. Furthermore, the distribution of xylem and stomata in the leaf showed evidence of coordinated development, as species with larger leaf total stomatal area also presented larger midrib vessel diameters (dh1) and higher midrib vein area (VA1), indicating that development is integrated to optimize water transport through leaf xylem and transpiration through stomata. Overall, species with lower leaf longevity (LL) and canopy foliage duration (FD), which also displayed traits related to a fast resource acquisition strategy (low SSD and LMA), have a higher stomatal index and free terminal vein density compared to species with higher LL and FD, which corresponded to species bearing more conservative acquisition traits (high SSD and LMA). The hydroscape area, reflecting whether species ranged from iso to anisohydry, was more strongly related to anatomical variation within the petiole and the primary and secondary veins than to stomatal traits or terminal vein traits. Results integrate leaf and stem anatomical characteristics with carbon and water resource use strategies in Sonoran Desert trees.
Interspecific traits variation
Leaf area varied significantly between species, and the leaf major veins traits were related to leaf area as has been shown before (Fig. 1, Table S1, Supplementary material, Sack et al. 2012). Therefore, we expected interspecific variation in the vessel diameter for primary and secondary veins, however, these traits did not present significant differences between species. In contrast, dhP presented significant differences between species, which could be related to greater investment in the petiole area to support larger leaves in comparison to smaller leaves (Sack et al. 2013). In addition, traits related to the number of terminal veins (TV/A and LTVN) also presented differences between the 10 species. A higher number of terminal veins is related to a higher vessel length per unit area, a trait that has been shown to vary between angiosperm species (Sack and Scoffoni 2013).
Leaf vessel scaling and its relationship to leaf area
We found a relationship between the scaling of the leaf vein and stomatal traits that was not directly related to the leaf area. However, we found that species with smaller petiole vessel diameter (dhP) presented lower values of vessel scaling (α), and species with higher dhP presented higher vessel widening from the terminal veins to the petiole (Table S1, Supplementary material). Also, species with higher dhP have a higher leaf area (Fig. 1). This indicates that species with larger vessel tapering from the petiole to the terminal veins present a higher water transport at the petiole level and a higher reduction in their vessel diameters and vein area to reach the mesophyll and the stomata. This reflects a strategy to maintain constant leaf hydraulic and photosynthetic productivity per leaf area as found by Echeverria et al. (2019). In addition, to high dhP, we found that species with higher leaf area presented higher dh1, dh2, and higher VAP, VA1, and VA2 (Fig. 1), traits related to a more efficient and faster water transport, indicating that species must regulate the leaf hydraulic system per leaf area. Species with larger areas must cover a larger surface to reach all the mesophyll tissue and, therefore, they present petiole, primary, and secondary veins with larger areas and larger vessel diameters. This relation allows constant leaf-specific conductance independently of the changes in leaf area. This relation has also been observed in interspecific studies of seedlings of species from Mediterranean vegetation (Zhong 2019, 2020) as well as intraspecific studies of species of the tropical dry forest (Echeverria et al. 2019). Therefore, there seems to be an indirect relation between the leaf size and stomata reflected by the strong coordination between water supply and demand. Interestingly, studies considering the whole plant found that species with higher height also present higher total leaf area, and higher widening of vein and vessel diameters (Echeverria et al. 2019, Zhong et al. 2019; Soriano et al. 2020; Olson et al. 2021). This dynamic is considered a strategy to maintain a constant water supply and demand, as well as constant CO2 assimilation, despite size changes at tree, stem, and leaf levels (Echeverria et al. 2019, Olson et al. 2021).
We did not find a correlation between vessel scaling α and leaf area, these could be related to atypical values of α for some species. For example, Bula, Olte, and Pami presented terminal conduits with higher area and diameter than vessels of secondary veins (Fig, S1 Supplementary Material), which for Olte and Pami can be related to their small leaf areas and might have been in the limit of the resolution of our imaging survey. However, Bula does not have small leaves, and for the tree species, we found storage tracheids at the end of the terminal veins that may serve as water reservoirs (Fig. S2 Supplementary Material, Evert 2006), thus probably enhancing the diameter. We did not find a significant correlation between stomatal area and with leaf area. We expected that the species with higher leaf area would present higher stomatal cell area which could reflect a higher transpirational area related to a higher transpirational demand in larger leaves (Lawson and Matthews 2020). However, there are other anatomical characteristics that are related to the transpirational area of the leaf, such as the number of stomata in the leaf (Zhang et al. 2022). For example, Sapindus saponaria, the species with the largest leaf area (6.71 cm2) presented the smallest stomata cell area (842 µm2) but presented a higher-than-average leaf stomatal cell number (477,499 stomata per leaf) which is related to a higher transpirational area.
Leaf vein xylem traits are coordinated with stomatal traits
Throughout evolutionary time, angiosperm species have presented a balance between water supply (represented by leaf xylem traits) and water demand (represented by stomatal traits) which remains constant in different lifeforms from different environments (Brodribb et al. 2017; Zhong et al. 2020; Wen et al. 2020; Zhang et al. 2022). In our study, we found that leaf stomatal number and leaf total stomatal area were both correlated with several major vein traits (i.e., vascular bundle area of the petiole, midrib, and secondary vein, Fig. 2). Leaf total stomatal area was also correlated with the hydraulic vessel diameter of the petiole, midrib, and secondary vein. The coordination between major vein traits and stomata indicates that species with larger leaves and, therefore, higher stomatal number and area, present a larger xylem conduct widening from the minor veins to the major veins and petiole. This could be seen as a strategy to reduce water transport resistance in leaves with larger areas of transpiration, and therefore, larger water demand as seen in seedlings of Mediterranean vegetation in Zhong et al. (2019, 2020).
Stomatal traits were also correlated to minor vein traits. Leaf stomatal number and area were both correlated with the leaf total terminal vein number, moreover, leaf total stomatal area was correlated with leaf total areola number. In addition, the stomatal area was correlated with the terminal vein area and vessel diameter. The relationship between stomatal traits and minor veins has been found in many environments and reflects a strong coordination between the leaf development and the environment as a differential cell expansion has a similar effect on the density of the xylem and stomata (Carins-Murphy et al. 2016, Brodribb et al. 2017; Zhang et al. 2022). Even though our species coexisted in the same environment, the different xylem and stomata densities indicate different evolutionary adaptations to the same environment (Brodribb and Field 2010) and reflect different strategies to balance water demand and supply.
Hydraulic leaf traits and the iso-anisohydric continuum and the plant economics spectrum
Species diverged in their leaf vein and stomatal traits in concert with their water and carbon resource use strategies; with anatomical traits being coordinated with traits associated with the iso-anisohydric continuum and the PES. We found that more anisohydric species (higher hydroscape area, HA, Fu and Meinzer et al. 2019) presented lower vessel diameters of the petiole, midrib, and secondary vein vessel (Fig. 3). This coincides with studies in temperate and arid vegetation in which more anisohydric species presented resource conservation traits such as greater embolism resistance and lower water conductance (Fu et al. 2019; Chen et al. 2021). In addition, species with stem traits related to slower resource use (reflected by a higher stem density, Reich 2014) present lower vessel diameters of the petiole, primary and secondary veins. This association between stem density and the overall hydraulic capability of the leaf xylem agrees with the idea that hydraulic and functional traits should be coordinated in leaves and stems, as posited by the plant economic spectrum (Méndez-Alonzo et al 2012; Reich 2014). We found that fast-growing species with low stem densities presented an efficient water transport (reflected by a wide vessel diameter) and slow-growing species with high tissue density presented a slower water transport (reflected by a narrow vessel diameter, Reich 2014; Zhong et al. 2019). Contrary to what we expected, species with leaf traits related to slower resource use (reflected by higher leaf mass per area, Reich 2014) presented higher terminal vein area. However, as explained before, some species normally considered as slow growing (Olte and Pami) presented large water storage tracheids in the terminal veins (Fig. S1, supplementary material) and therefore this species could be affecting the correlation with LMA.
Our synthetic ordination analysis across species shows that traits in the more isohydric species, such as Jaco, Bufa, Foma, and Bula, could reflect a faster acquisition of resources with stems and leaves of low construction costs (with lower LMA) and vessels in leaves that allow them to transport water faster to the transpiration area (see Chen et al. 2021, Fig. 5). However, these species also are less resistant to drought since a higher conduct diameter enhances their sensitivity to xylem embolisms (Sack and Scoffoni 2013). On the other extreme, the species tending towards anisohydry, such as Boma, Guco, and Olte, presented higher stem-specific density and leaf mass per area but lower midrib/petiole vessel diameter, leaf area, and petiole vessel bundle area. As expected, higher stem-specific density and leaf mass per area imply higher construction cost, more long-term carbon investment, and therefore, higher leaf longevity and mechanical strength (Chave et al. 2009; Wright et al. 2004). Narrower xylem conduct diameter and vein area are related to lower hydraulic transport and less risk of embolism formation (Sack and Scoffoni 2013). They might be a correlative consequence of the specific tendency towards miniaturization of the hydraulic pathways. These minute leaves are, therefore, more resistant to embolism formation which probably helps them be more stress-resistant (Chen et al. 2021).
On the second principal component, species differentiated with respect to phenology (Fig. 5). Trees with higher leaf longevity (LL) and higher foliage duration (FD) in the canopy had a lower density of free terminal veins (TV/A) and a lower stomatal index (SI), such as Boma, Guco, and Olte. A higher StoI is related to a lower epidermal cell area and higher stomatal density (Sack and Buckley 2016). Leaves with higher StoI and stomata density tend to have smaller stomata which have faster rates of stomata opening, and therefore, higher rates of CO2 assimilation and stomatal conductance than species with lower StoI and stomatal density (Drake et al. 2013, Lawson and Matthews 2020). In addition, species with higher free-ending veins per area (Lydi, Pami) have previously been related to a higher vein length per area (Sack et al. 2015) which is related to a higher xylem surface area and a higher leaf hydraulic conductivity (Sack and Scoffoni 2013; Sack et al. 2015). Therefore, Boma, Guco, and Olte, the species with higher LL and FD presented traits related to a lower stomatal conductance and leaf hydraulic conductance and, therefore, a more conservative resource use strategy that allows them to resist the long periods of drought (Sack et al. 2013; Sack and Buckley 2016; Brodribb et al. 2017). In contrast, trees with lower FD and LL present a higher TV/A and a higher StoI which could reflect a higher maximum stomatal conductance and higher leaf hydraulic conductivity, promoting faster water transport and larger carbon assimilation rates during the short period that they have leaves (Sack et al.2013; Sack and Buckley 2016; Brodribb et al. 2017). Therefore, species are distributed in a gradient between slow to fast water and carbon resource acquisition in the two principal components.
Conclusions
The 10 Sonoran Desert tree species present a diversity of hydraulic traits that reflect a balance between water supply and demand. Leaf xylem vessels are correlated with leaf area which reflects a constant water supply to the leaves independently of their leaf size. In addition, traits of the leaf minor and major veins were coordinated with stomatal traits related to the leaf transpiration demand. Furthermore, tree species of the Sonoran Desert follow an anatomical and physiological axis, ranging from exploitative to conservative species, in which fast-deciduous species are characterized by high diameter leaf vein xylem and high stomatal sizes, turning them to be also prone to display higher hydraulic conductance and gas exchange rates in comparison with slow-evergreen species, which tend towards the opposite values of these characters. In conjunction, our dataset supports the notion that frost-free communities of the Sonoran Desert bear a wide range of xylem and stomata characteristics that generate a water provision-transpiration balance and allow these species to achieve permanence under extremely seasonal environments.
References
Adams DC, Collyer ML (2018) Phylogenetic ANOVA: group-clade aggregation, biological challenges, and a refined permutation procedure. Evolution 72:1204–1215. https://doi.org/10.1111/evo.13492
Brito-Castillo L, Crimmins MA, Díaz SC (2010) Clima. In: Molina-Freaner FE, Van Devender TR (eds) Diversidad biológica de Sonora. Universidad Nacional Autónoma de México, Mexico City, pp 73–96
Brodribb TJ, Feild TS (2010) Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol Lett 13:175–183. https://doi.org/10.1111/j.1461-0248.2009.01410.x
Brodribb TJ, McAdam SA, Carins Murphy MR (2017) Xylem and stomata, coordinated through time and space. Plant Cell Environ 40:872–880. https://doi.org/10.1111/pce.12817
Carins Murphy MR, Jordan GJ, Brodribb TJ (2016) Cell expansion not cell differentiation predominantly co-ordinates veins and stomata within and among herbs and woody angiosperms grown under sun and shade. Ann Bot 118:1127–1138. https://doi.org/10.1093/aob/mcw167
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366. https://doi.org/10.1111/j.1461-0248.2009.01285.x
Chen Z, Zhang Y, Yuan W, Zhu S, Pan R, Wan X, Liu S (2021) Coordinated variation in stem and leaf functional traits of temperate broadleaf tree species in the isohydric–anisohydric spectrum. Tree Physiol 41:1601–1610. https://doi.org/10.1093/treephys/tpab028
Coomes DA, Heathcote S, Godfrey ER, Shepherd JJ, Sack L (2008) Scaling of xylem vessels and veins within the leaves of oak species. Biol Lett 4:302–306. https://doi.org/10.1098/rsbl.2008.0094
Dimmitt MA (2015) Plant Ecology of the Sonoran Desert Region. In: Dimmitt MA, Comus PW, Phillips SJ, Brewer LM (eds) A natural history of the Sonoran Desert. Univ of California Press, Tucson
Echeverría A, Anfodillo T, Soriano D, Rosell JA, Olson ME (2019) Constant theoretical conductance via changes in vessel diameter and number with height growth in Moringa oleifera. J Exp Bot 70:5765–5772. https://doi.org/10.1093/jxb/erz329
ElDrake PL, Froend RH, Franks PJ (2013) Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot 64:495–505. https://doi.org/10.1093/jxb/ers347
El-Sharkawy MA, Cock JH, Hernandez AP (1985) Stomatal response to air humidity and its relation to stomatal density in a wide range of warm climate species. Photosynth Res 7:137–149. https://doi.org/10.1007/BF00037004
Evert RF (2006) Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. John Wiley & Sons, New Jersey
Ezcurra E, Rodrigues V (1986) Rainfall patterns in the gran Desierto, Sonora, Mexico. J Arid Environ 10:13–28. https://doi.org/10.1016/S0140-1963(18)31261-8
CESAVE-SIAFESON (2019) Red de estaciones meteorológicas automáticas de sonora www.siafeson.com/remas (19 September 2023, date last accessed).
Fu X, Meinzer FC (2019) Metrics and proxies for stringency of regulation of plant water status (iso/anisohydry): a global data set reveals coordination and trade-offs among water transport traits. Tree Physiol 39:122–134. https://doi.org/10.1093/treephys/tpy087
Fu X, Meinzer FC, Woodruff DR, Liu YY, Smith DD, McCulloh KA, Howard AR (2019) Coordination and trade-offs between leaf and stem hydraulic traits and stomatal regulation along a spectrum of isohydry to anisohydry. Plant Cell Environ 42:2245–2258. https://doi.org/10.1111/pce.13543
Garland T Jr, Dickerman AW, Janis CM, Jones JA (1993) Phylogenetic analysis of covariance by computer simulation. Syst Biol 42:265–292. https://doi.org/10.1093/sysbio/42.3.265
González-Medrano, F. (2012). Las zonas áridas y semiáridas de México y su vegetación. Instituto Nacional de Ecología, Ciudad de México.
Gonzalez-Rebeles G, Terrazas T, Mendez-Alonzo R, Paz H, Brodribb TJ, Tinoco-Ojanguren C (2021) Leaf water relations reflect canopy phenology rather than leaf life span in Sonoran Desert trees. Tree Physiol 41:1627–1640. https://doi.org/10.1093/treephys/tpab032
Gonzalez-Rebeles G, Mendez-Alonzo R, Paz H, Terrazas T, Tinoco-Ojanguren C (2023) Hydraulic strategies in evergreen and deciduous tree saplings from the Sonoran Desert. Tree Physiol. https://doi.org/10.1093/treephys/tpac114
IPBES (2018) The IPBES assessment report on land degradation and restoration. In: Montanarella L, Scholes R, Brainich A (eds) Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Bonn, Germany, p 744
Lambers H, Oliveira RS (2019) Plant Physiological Ecology. Springer, Cham
Lawson T, Matthews J (2020) Guard cell metabolism and stomatal function. Annu Rev Plant Biol 71:273–302. https://doi.org/10.1146/annurev-arplant-050718-100251
Li F, McCulloh KA, Sun S, Bao W (2018) Linking leaf hydraulic properties, photosynthetic rates, and leaf lifespan in xerophytic species: a test of global hypotheses. Am J Bot 105:1858–1868. https://doi.org/10.1002/ajb2.1185
Martínez-Cabrera D, Terrazas T, Ochotorena H (2009) Foliar and petiole anatomy of tribe Hamelieae and other Rubiaceae. Ann Mo Bot Gard 96:133–145. https://doi.org/10.3417/2006196
Meinzer FC, Woodruff DR, Marias DE, Smith DD, McCulloh KA, Howard AR, Magedman AL (2016) Mapping ‘hydroscapes’ along the iso-to anisohydric continuum of stomatal regulation of plant water status. Ecol Lett 19:1343–1352. https://doi.org/10.1111/ele.12670
Melgarejo LM (ed) (2010) Experimentos en Fisiología Vegetal. Universidad Nacional de Colombia, Bogota
Méndez-Alonzo R, Paz H, Zuluaga RC, Rosell JA, Olson ME (2012) Coordinated evolution of leaf and stem economics in tropical dry forest trees. Ecology 93:2397–2406. https://doi.org/10.1890/11-1213.1
Navea C, Terrazas T, Delgado-Salinas A, Ramírez-Vallejo P (2002) Foliar response of wild and domesticated Phaseolus vulgaris L. to water stress. Genet Resour Crop Evol 49:125–132. https://doi.org/10.1023/A:1014727302512
Niinemets Ü, Portsmuth A, Tobias M (2006) Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytol 171:91–104. https://doi.org/10.1111/j.1469-8137.2006.01741.x
Olson ME, Anfodillo T, Gleason SM, McCulloh KA (2021) Tip-to-base xylem conduit widening as an adaptation: causes, consequences, and empirical priorities. New Phytol 229:1877–1893. https://doi.org/10.1111/nph.16961
Reich PB (2014) The world-wide ‘fast–slow’plant economics spectrum: a traits manifesto. J Ecol 102:275–301. https://doi.org/10.1111/1365-2745.12211
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, Oxford
Sack L, Buckley TN (2016) The developmental basis of stomatal density and flux. Plant Physiol 171:2358–2363. https://doi.org/10.1104/pp.16.00476
Sack L, Holbrook NM (2006) Leaf hydraulics. Annu Rev Plant Biol 57:361–381. https://doi.org/10.1146/annurev.arplant.56.032604.144141
Sack L, Scoffoni C (2013) Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol 198:983–1000. https://doi.org/10.1111/nph.12253
Sack L, Tyree MT (2005) Leaf hydraulics and its implications in plant structure and function. In: Holbrook NM, Zwieniecki MA (eds) Vascular transport in plants. Academic Press, Burlington, pp 93–114
Sack L, Scoffoni C, McKown AD, Frole K et al (2012) Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat Commun 3:1–10. https://doi.org/10.1038/ncomms1835
Sack L, Scoffoni C, John GP, Poorter H, Mason CM, Mendez-Alonzo R, Donovan LA (2013) How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J Exp Bot 64:4053–4080. https://doi.org/10.1093/jxb/ert316
Sack L, Scoffoni C, Johnson DM, Buckley TN, Brodribb TJ (2015) Chapter 10: anatomical determinants of leaf hydraulic function. In: Hacke UG (ed) Functional and ecological xylem anatomy. Springer, Cham, pp 255–271
Salisbury EJ (1927) On the causes and ecological significance on stomatal frequency with special reference to woodland flora. Phil Trans Roy Soc Lond Ser B 216:1–65
Shreve F (1951) Vegetation of the Sonoran Desert. Carnegie Institution of Washington, Washington, DC
Shreve F, Wiggins IL (1964) Vegetation and flora of the Sonoran Desert. Stanford University Press, Stanford
Soriano D, Echeverría A, Anfodillo T, Rosell JA, Olson ME (2020) Hydraulic traits vary as the result of tip-to-base conduit widening in vascular plants. J Exp Bot 71:4232–4242. https://doi.org/10.1093/jxb/eraa157
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviors. J Exp Bot 49:419–432. https://doi.org/10.1093/jxb/49.Special_Issue.419
Terrazas T, Aguilar-Rodríguez S, Tinoco-Ojanguren C (2011) Development of successive cambia, cambial activity, and their relationship to physiological traits in Ipomoea arborescens (Convolvulaceae) seedlings. Am J Bot 98:765–774. https://doi.org/10.3732/ajb.1000182
Turner RM, Bowers JE, Burgess TL (1995) Sonoran Desert plants: an ecological atlas. The University of Arizona Press, Tucson
Volaire F (2018) A unified framework of plant adaptive strategies to drought: crossing scales and disciplines. Global Chang Biol 24:2929–2938. https://doi.org/10.1111/gcb.14062
Wen Y, Zhao WL, Cao KF (2020) Global convergence in the balance between leaf water supply and demand across vascular land plants. Funct Plant Biol 47:904–911. https://doi.org/10.1071/fp19101
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Zhang M, Gao H, Chen S, Wang X, Mo W, Yang X et al (2022) Linkages between stomatal density and minor leaf vein density across different altitudes and growth forms. Front Plant Sci 13:1064344. https://doi.org/10.3389/fpls.2022.1064344
Zhong M, Castro-Díez P, Puyravaud JP, Sterck FJ, Cornelissen JH (2019) Convergent xylem widening among organs across diverse woody seedlings. New Phytol 222:1873–1882. https://doi.org/10.1111/nph.15734
Zhong M, Cerabolini BE, Castro-Díez P, Puyravaud JP, Cornelissen JH (2020) Allometric co-variation of xylem and stomata across diverse woody seedlings. Plant Cell Environ 43:2301–2310. https://doi.org/10.1111/pce.13826
Acknowledgements
This study is a partial fulfillment of the requirements for GGR to obtain the Doctor of Science degree in the Posgrado en Ciencias Biológicas (PCB) at the Universidad Nacional Autónoma de México (UNAM). The authors thank Alicia Rojas, Jose F. Martínez, and Anabel Díaz Martínez for field and laboratory assistance, to the authorities of the Centro Ecológico del Estado de Sonora (CEES) and Instituto Nacional de Antropología e Historia (INAH) Sonora for access to their properties. We thank Ginna E. Fernández for assistance with the Hydroscape Area. We also thank Dulce Olivia Espinoza, Sharon Hernandez, Fernanda Nuñez, Gabriela Blanco, Alejandro Bolado, and Claudia Canales for field assistance. We thank Julieta Rosell García, Sara Ceccarelli Fadia, Ricardo Sánchez-Martín for their assistance with the phylogenetic ANOVA. G.G.-R and C.T.O. thanks Consejo Nacional de Humanidades Ciencias y Tecnologías (Grant No.280508) and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, UNAM (PAPIIT IN212220) for their financial support.
Funding
Consejo Nacional de Humanidades Ciencias y Tecnologías (Conahcyt), Grant No. 280508 (GGR) and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-UNAM), Grant No. IN212220 (CT).
Author information
Authors and Affiliations
Contributions
GG-R, CT-O, RM-A, and TT conceived and designed the study; GG-R, CT-O, and TT participated in the experiment execution and collection of data; GG-R, CT-O, RM-A, and TT contributed to data interpretation and manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Communicated by Jesus Julio Camarero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonzalez-Rebeles, G., Terrazas, T., Mendez-Alonzo, R. et al. Coordination of hydraulic and functional traits in ten species of trees of the Sonoran Desert. Trees 37, 1743–1756 (2023). https://doi.org/10.1007/s00468-023-02456-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02456-8