Abstract
Key message
Our study elucidated that Q. dentata responds to Na2CO3 stress by excreting salt on the blade, accumulating osmotic protectants, and changing the cell ultrastructure and physiological-biochemical parameters of leaves.
Abstract
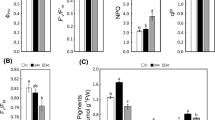
Quercus dentata Thunb is an important constructive species in the forest communities of the mountainous areas of northern China with high ecological and landscape value. However, soil salinization prevents the use of Q. dentata in the North China Plain. Therefore, it is necessary to explore how Q. dentata responds to saline-alkaline stress. Here, Q. dentata seedlings were exposed to different concentrations of an alkaline salt (Na2CO3) to determine the effects on leaf mesophyll cell ultrastructure and physiological-biochemical parameters. We first discovered crystallized deposits on the pressure side of Q. dentata leaf blades after the 100 mM Na2CO3 treatment. Scanning electron microscopy and energy-dispersive X-ray analysis revealed that the main component of the crystal deposits was sodium ions. However, no salt glands or bladders were detected in Q. dentata leaves. Moreover, the ultrastructure of the mesophyll cells changed as stress duration increased, and the number and size of the chloroplasts were limited by partial disintegration. The results also show that Na2CO3-stressed Q. dentata plants displayed increased soluble sugar and proline contents. Additionally, the low-concentration Na2CO3 stress slightly enhanced the activity of antioxidant enzymes, such as catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD). In summary, the results show that the cell structure of Q. dentata leaves was damaged by Na2CO3 stress. However, accumulating osmoprotectants and the excretion of salt onto the leaf blades enhanced saline-alkaline stress tolerance.








Similar content being viewed by others
Data availability
Data in this study are available from the corresponding authors upon reasonable request.
References
Al-Harrasi A, Rehman NU, Khan AL, Al-Broumi M, Al-Amri I, Hussain J, Hussain H, Csuk R (2018) Chemical, molecular and structural studies of Boswellia species: β-Boswellic Aldehyde and 3-epi-11β-dihydroxy BA as precursors in biosynthesis of boswellic acids. PLoS ONE 13:e0198666
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53:1367–1376
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Cao L, Weinan C, Zenghui H, Pingsheng L (2018) Effect of saline-alkaline stress on the growth and photosynthetic characteristics of Quercus dentata seedlings. J Beijing Agric Coll 33:86–90
Chuamnakthong S, Nampei M, Ueda A (2019) Characterization of Na(+) exclusion mechanism in rice under saline-alkaline stress conditions. Plant Sci 287:110171
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Denk T, Grimm GW, Manos PS, Deng M, Hipp AL (2017) An updated infrageneric classification of the oaks: review of previous taxonomic schemes and synthesis of evolutionary patterns. In: Gil-Pelegrín E, Peguero-Pina JJ, Sancho-Knapik D (eds) Oaks physiological ecology exploring the functional diversity of genus quercus L. Cham: Springer International Publishing 13–38
Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, Palmgren MG, Zhu JK (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ -ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19:1617–1634
Guo R, Shi L, Ding X, Hu Y, Tian S, Yan D, Shao S, Gao Y, Liu R, Yang Y (2010) Effects of saline and alkaline stress on germination, seedling growth, and ion balance in wheat. Agron J 102:1252–1260
Guo R, Shi L, Yan C, Zhong X, Gu F, Liu Q, Xia X, Li H (2017) Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol 17:41
Hartung W, Leport L, Ratcliffe RG, Sauter A, Duda R, Turner NC (2002) Abscisic acid concentration, root pH and anatomy do not explain growth differences of chickpea (Cicer arietinum L.) and lupin (Lupinus angustifolius L.) on acid and alkaline soils. Plant Soil 240:191–199
Hasanuzzaman M, Davies NW, Shabala L, Zhou M, Brodribb TJ, Shabala S (2017) Residual transpiration as a component of salinity stress tolerance mechanism: a case study for barley. BMC Plant Biol 17:107
He J, Chen F, Chen S, Lv G, Deng Y, Fang W, Liu Z, Guan Z, He C (2011) Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J Plant Physiol 168:687–693
Irigoyen JJ, Einerich DW, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol Plant 84:55–60
Julkowska MM, Testerink C (2015) Tuning plant signaling and growth to survive salt. Trends Plant Sci 20:586–594
Kim KW, Koo K, Kim PG (2011) Seawater spray injury to Quercus acutissima leaves: crystal deposition, stomatal clogging, and chloroplast degeneration. Microsc Res Tech 74:449–456
Kosma DK, Murmu J, Razeq FM, Santos P, Bourgault R, Molina I, Rowland O (2014) AtMYB41 activates ectopic suberin synthesis and assembly in multiple plant species and cell types. Plant J 80:216–229
Kotula L, Clode PL, Jimenez JC, Colmer TD (2019) Salinity tolerance in chickpea is associated with the ability to ‘exclude’ Na from leaf mesophyll cells. J Exp Bot 70:4991–5002
Labidi N, Ammari M, Mssedi D, Benzerti M, Snoussi S, Abdelly C (2010) Salt excretion in Suaeda fruticosa. Acta Biol Hung 61:299–312
Li Z, Zhang WH (2013) Growth and physiological response of Quercus variabilis seedlings under NaCl stress. Acta Botan Boreali-Occiden Sin 33:1630–1637
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
More P, Agarwal P, Joshi PS, Agarwal PK (2019) The JcWRKY tobacco transgenics showed improved photosynthetic efficiency and wax accumulation during salinity. Sci Rep 9:19617
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Munns R, James RA, Gilliham M, Flowers TJ, Colmer TD (2016) Tissue tolerance: an essential but elusive trait for salt-tolerant crops. Funct Plant Biol FPB 43:1103–1113
Natali L, Vangelisti A, Guidi L, Remorini D, Cotrozzi L, Lorenzini G, Nali C, Pellegrini E, Trivellini A, Vernieri P, Landi M, Cavallini A, Giordani T (2018) How Quercus ilex L. saplings face combined salt and ozone stress: a transcriptome analysis. BMC Genom 19:872
Niu M, Xie J, Chen C, Cao H, Sun J, Kong Q, Shabala S, Shabala L, Huang Y, Bie Z (2018) An early ABA-induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J Exp Bot 69:4945–4960
Pankova E, Vorobieva L, Balyuk S, Khasankhanova G, Konyushkova M, Yamnova I. (2018) Salt-affected soils of the Eurasian region: diagnostics, criteria and distribution. Handbook for saline soil management 3–15
Paz RC, Rocco RA, Reinoso H, Menéndez AB, Pieckenstain FL, Ruiz OA (2012) Comparative study of alkaline, saline, and mixed saline-alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of Lotus tenuis. J Plant Growth Regul 31:448–459
Reinoso H, Sosa L, Ramírez L, Luna V (2004) Salt-induced changes in the vegetative anatomy of Prosopis strombulifera (Leguminosae). Can J Bot 82:618–628
Santos J, Al-Azzawi M, Aronson J, Flowers TJ (2016) eHALOPH a database of salt-tolerant plants: helping put halophytes to work. Plant Cell Physiol 57:e10
Shen Q, Yu J, Fu L, Wu L, Dai F, Jiang L, Wu D, Zhang G (2018) Ionomic, metabolomic and proteomic analyses reveal molecular mechanisms of root adaption to salt stress in tibetan wild barley. Plant Physiol Biochem 123:319–330
Suo J, Zhang H, Zhao Q, Zhang N, Zhang Y, Li Y, Song B, Yu J, Cao J, Wang T, Luo J, Guo L, Ma J, Zhang X, She Y, Peng L, Ma W, Guo S, Miao Y, Chen S, Qin Z, Dai S (2020) Na(2)CO(3)-responsive photosynthetic and ROS scavenging mechanisms in chloroplasts of alkaligrass revealed by phosphoproteomics. Genom Proteom Bioinform 18:271–288
van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71:403–433
Xu W, Jia L, Baluška F, Ding G, Shi W, Ye N, Zhang J (2012) PIN2 is required for the adaptation of Arabidopsis roots to alkaline stress by modulating proton secretion. J Exp Bot 63:6105–6114
Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217:523–539
Yang C, Chong J, Li C, Kim C, Shi D, Wang D (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294:263–276
Yang C, Shi D, Wang D (2008) Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul 56:179–190
Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Chen S, Fuglsang AT, Palmgren MG, Schumaker KS, Deng XW, Guo Y (2010) The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 22:1313–1332
Yuan F, Leng B, Wang B (2016) Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front Plant Sci 7:977
Zhang CL, Hu X, Zhang YL, Liu Y, Wang GL, You CX, Li YY, Hao YJ (2020) An apple long-chain acyl-CoA synthetase 2 gene enhances plant resistance to abiotic stress by regulating the accumulation of cuticular wax. Tree Physiol 40:1450–1465
Zhao Q, Suo J, Chen S, Jin Y, Ma X, Yin Z, Zhang Y, Wang T, Luo J, Jin W, Zhang X, Zhou Z, Dai S (2016) Na2CO3-responsive mechanisms in halophyte Puccinellia tenuiflora roots revealed by physiological and proteomic analyses. Sci Rep 6:32717
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Acknowledgements
This study was supported by the Innovative Transdisciplinary Program of Ecological Restoration Engineering under Beijing Municipal Education Commission (IDHT20190509), Scientific Research Project of Beijing Educational Committee (SQKM20181002015), and the grant for Beijing Forestry University Outstanding Postgraduate Mentoring Team Building (YJSY-DSTD2022005). We thank Editorbar Language Editing for editing the English text of a draft of this manuscript.
Funding
Beijing Forestry University Outstanding Postgraduate Mentoring Team Building, YJSY-DSTD2022005, Cunfu Lu, Innovative Transdisciplinary Program of Ecological Restoration Engineering under Beijing Municipal Education Commission,IDHT20190509, Scientific Research Project of Beijing Educational Committee, SQKM20181002015.
Author information
Authors and Affiliations
Contributions
PSL, CFL, and ZHH conceived and designed the study; WBW, LC, and WNC prepared the materials, conducted the experiments, WBW analyzed the data and prepared the results; WBW wrote the manuscript; PSL, ZHH, and CFL edited and improved the manuscript; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Communicated by Jinxing Lin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, WB., Cao, L., Chen, W. et al. Quercus dentata responds to Na2CO3 stress with salt crystal deposits: ultrastructure, and physiological–biochemical parameters of leaves. Trees 37, 1001–1011 (2023). https://doi.org/10.1007/s00468-023-02400-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02400-w