Abstract
Key message
In Pinus radiata, both primary and secondary compounds may play a role in defence against herbivory. Allocation of constitutive and induced primary and secondary compounds varies among needles, bark and roots. Responses to stresses are stronger in primary than secondary compounds and some responses of root chemistry to above-ground stressors were detected.
Abstract
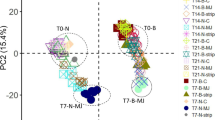
The capacity of trees to cope with pests and pathogens depends in part on the variation of constitutive and induced chemical defences within the plant. Here we examined the constitutive and induced variation of primary (sugars and fatty acids) and secondary (mono-, sesqui- and di- terpenoids as well as volatile phenolics) metabolites in the needles, bark and, for the first time, roots of 2-year old Pinus radiata. A total of 81 compounds were examined. The plant parts differed significantly in constitutive levels of individual sugars, fatty acids, mono-, sesqui- and di- terpenoids as well as volatile phenolics. Overall, the bark had more compounds and a higher amount of most secondary compounds and the levels of compounds in the roots differed from that of the needles and bark. For example, glucose was the dominant sugar in the needles and bark whereas fructose dominated in the roots. Of the fully identified secondary compounds, monoterpenoids dominated in all plant parts but with different qualitative patterns. Following methyl jasmonate and bark stripping treatments, a marked reduction in sugars but weaker changes in secondary compounds were detected in the needles and bark. Responses in the roots were minor but the few that were detected were mostly in response to the bark stripping treatment. Changes in correlations among chemicals within plant parts and between the same compound across the different plant parts were also detected after stress treatments. Overall, results showed that the constitutive composition in the roots differs from that of the bark and needles in P. radiata and inducibility is stronger in the primary than secondary metabolites and differs between plant parts. This detailed assessment of Pinus radiata chemistry in the needles, bark and roots, including the compounds that respond to simulated biotic stress will potentially facilitate the identification of related chemical defence traits.




Similar content being viewed by others
Availability of data and material
Not applicable.
References
Apetrei CL, Tuchilus C, Aprotosoaie AC, Oprea A, Malterud KE, Miron A (2011) Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules 16:7773–7788
Arhipova N, Jansons A, Zaluma A, Gaitnieks T, Vasaitis R (2015) Bark stripping of Pinus contorta caused by moose and deer: wounding patterns, discoloration of wood, and associated fungi. Can J for Res 45:1434–1438
Axelrod D (1988) Paleoecology of a Late Pleistocene Monterey pine at Laguna Niguel, southern California. Int J Plant Sci 149:458–464
Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, Thorpe MR, Orians CM (2005) Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol 167:63–72
Babst BA, Ferrieri RA, Thorpe MR, Orians CM (2008) Lymantria dispar herbivory induces rapid changes in carbon transport and partitioning in Populus nigra. Entomol Exp Appl 128:117–125
Barton KE, Koricheva J (2010) The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am Nat 175:481–493
Bonello P, Gordon TR, Storer AJ (2001) Systemic induced resistance in Monterey pine. Forest Pathol 31:99–106
Brockerhoff E, Bulman L (2014) Biosecurity risks to New Zealand’s plantation forests and the rationale for pathway risk management. N Z J for 59:3–8
Bucyanayandi JD, Bergeron J-M, Menard H (1990) Preference of meadow voles (Microtus pennsylvanicus) for conifer seedlings: chemical components and nutritional quality of bark of damaged and undamaged trees. J Chem Ecol 16:2569–2579
Burdon RD, Libby WJ, Brown AG (2017) Domestication of Radiata Pine. Springer Nature, Cham
Clancy KM (1992) The role of sugars in western spruce budworm nutritional ecology. Ecol Entomol 17:189–197
Cool LG, Zavarin E (1992) Terpene variability of mainland Pinus radiata. Biochem Syst Ecol 20:133–144
Cranswick AM, Rook DA, Zabkiewicz JA (1987) Seasonal changes in carbohydrate concentration and composition of different tissue types of Pinus radiata trees. NZ J Forest Sci 17:229–245
De Coninck B, Timmermans P, Vos C, Cammue BP, Kazan K (2015) What lies beneath: belowground defense strategies in plants. Trends Plant Sci 20:91–101
Deslauriers A, Caron L, Rossi S (2015) Carbon allocation during defoliation: testing a defense-growth trade-off in balsam fir. Front Plant Sci 6
Dettlaff MA, Marshall V, Erbilgin N, Cahill JF, Jr. (2018) Root condensed tannins vary over time, but are unrelated to leaf tannins. AoB PLANTS 10, ply044-ply044.
Dobbelstein E, Fink D, Öner-Sieben S, Czempik L, Lohaus G (2019) Seasonal changes of sucrose transporter expression and sugar partitioning in common European tree species. Tree Physiol 39:284–299
Eveland AL, Jackson DP (2012) Sugars, signalling, and plant development. J Exp Bot 63:3367–3377
Eyles A, Smith D, Pinkard EA, Smith I, Corkrey R, Elms S, Beadle C, Mohammed C (2011) Photosynthetic responses of field-grown Pinus radiata trees to artificial and aphid-induced defoliation. Tree Physiol 31:592–603
Felicijan M, Novak M, Kraševec N, Urbanek Krajnc A (2015) Antioxidant defences of Norway spruce bark against bark beetles and its associated blue-stain fungus. Agricultura 12:9–18
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–376
Franich RA, Gadgil PD, Shain L (1983) Fungistatic effects of Pinus radiata needle epicuticular fatty and resin acids on Dothistroma pini. Physiol Plant Pathol 23:183–195
Frost CJ, Hunter MD (2008) Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol 178:835–845
Gershenzon J (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20:1281–1328
Goodsman DW, Lusebrink I, Landhäusser SM, Erbilgin N, Lieffers VJ (2013) Variation in carbon availability, defense chemistry and susceptibility to fungal invasion along the stems of mature trees. New Phytol 197:586–594
Gould N, Reglinski T, Spiers M, Taylor JT (2008) Physiological trade-offs associated with methyl jasmonate-induced resistance in Pinus radiata. Can J for Res 38:677–684
Gould N, Reglinski T, Northcott GL, Spiers M, Taylor JT (2009) Physiological and biochemical responses in Pinus radiata seedlings associated with methyl jasmonate-induced resistance to Diplodia pinea. Physiol Mol Plant Pathol 74:121–128
Hartmann H, Trumbore S (2016) Understanding the roles of nonstructural carbohydrates in forest trees—from what we can measure to what we want to know. New Phytol 211:386–403
Hernandez-Escribano L, Iturritxa E, Aragonés A, Mesanza N, Berbegal M, Raposo R, Elvira-Recuenco M (2018) Root infection of canker pathogens, Fusarium circinatum and Diplodia sapinea, in asymptomatic trees in Pinus radiata and Pinus pinaster plantations. Forests 9:128
Holopainen JK, Virjamo V, Ghimire RP, Blande JD, Julkunen-Tiitto R, Kivimäenpää M (2018) Climate change effects on secondary compounds of forest trees in the northern hemisphere. Front Plant Sci 9:1445
Huber DPW, Philippe RN, Madilao LL, Sturrock RN, Bohlmann J (2005) Changes in anatomy and terpene chemistry in roots of Douglas-fir seedlings following treatment with methyl jasmonate. Tree Physiol 25:1075–1083
Huot B, Yao J, Montgomery BL, He SY (2014) Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant 7:1267–1287
Iason GR, O’Reilly-Wapstra JM, Brewer MJ, Summers RW, Moore BD (2011) Do multiple herbivores maintain chemical diversity of Scots pine monoterpenes? Philos Trans R Soc B Biol Sci 366:1337–1345
Ilse LM, Hellgren EC (2007) Indirect interactions among dendrophages: Porcupines predispose pinyon pines to bark beetle attack. For Ecol Manag 242:217–226
Ishangulyyeva G, Najar A, Curtis JM, Erbilgin N (2016) Fatty acid composition of novel host jack pine do not prevent host acceptance and colonization by the invasive mountain pine beetle and its symbiotic fungus. PLoS ONE 11:e0162046
Jones TH, Potts BM, Vaillancourt RE, Davies NW (2002) Genetic resistance of Eucalyptus globulus to autumn gum moth defoliation and the role of cuticular waxes. Can J for Res 32:1961–1969
Kachroo A, Kachroo P (2009) Fatty acid–derived signals in plant defense. Annu Rev Phytopathol 47:153–176
Kajdzanoska M, Petreska J, Stefova M (2011) Comparison of different extraction solvent mixtures for characterization of phenolic compounds in strawberries. J Agric Food Chem 59:5272–5278
Kant MR, Jonckheere W et al (2015) Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann Bot 115:1015–1051
Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF (2008) Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 89:392–406
Kempel A, Schädler M, Chrobock T, Fischer M, van Kleunen M (2011) Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proc Natl Acad Sci 108:5685–5689
Kleine S, Müller C (2013) Differences in shoot and root terpenoid profiles and plant responses to fertilisation in Tanacetum vulgare. Phytochemistry 96:123–131
Kurek T, Todys J, Pazdrowski W, Szymański M, Łukowski A (2019) Intensity of stripping and sugar content in the bark and the bast of European beech (Fagus sylvatica). Open Life Sci 14:19–28
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Lewinsohn E, Gijzen M, Croteau R (1991) Defense mechanisms of conifers: differences in constitutive and wound-induced monoterpene biosynthesis among species. Plant Physiol 96:44
Li C-Y, Weiss D, Goldschmidt EE (2003) Girdling affects carbohydrate-related gene expression in leaves, bark and roots of alternate-bearing citrus trees. Ann Bot 92:137–143
Lombardero MJ, Pereira-Espinel J, Ayres MP (2013) Foliar terpene chemistry of Pinus pinaster and P. radiata responds differently to methyl jasmonate and feeding by larvae of the pine processionary moth. For Ecol Manag 310:935–943
Lombardero MJ, Ayres MP, Bonello P, Cipollini D, Herms DA (2016) Effects of defoliation and site quality on growth and defenses of Pinus pinaster and P. radiata. For Ecol Manag 382:39–50
López-Goldar X, Villari C, Bonello P, Borg-Karlson AK, Grivet D, Sampedro L, Zas R (2019) Genetic variation in the constitutive defensive metabolome and its inducibility are geographically structured and largely determined by demographic processes in maritime pine. J Ecol 107:2464–2477
López-Goldar X, Lundborg L, Borg-Karlson AK, Zas R, Sampedro L (2020) Resin acids as inducible chemical defences of pine seedlings against chewing insects. PLoS ONE 15:e0232692
López-Goldar X, Villari C, Bonello P, Borg-Karlson A-K, Grivet D, Zas R, Sampedro L (2018) Inducibility of plant secondary metabolites in the stem predicts genetic variation in resistance against a key insect herbivore in maritime pine. Front Plant Sci 9
Lundborg L, Sampedro L, Borg-Karlson A-K, Zas R (2019) Effects of methyl jasmonate on the concentration of volatile terpenes in tissues of Maritime pine and Monterey pine and its relation to pine weevil feeding. Trees 33:53–62
McDonald JH (2009) Handbook of biological statistics. In: 2nd edn. (Sparky House Publishing Baltimore)
McKey D (1974) Adaptive patterns in alkaloid physiology. Am Nat 108:305–320
McKey D (1979) The distribution of secondary compounds within plants. In 'Herbivores: Their interaction with secondary plant metabolites. 1st edn. (Eds GA Rosenthal, DH Jansen and SW Applebaum) pp. 56–133. (Academic Press: New York).
Mead DJ (2013) Sustainable management of Pinus radiata plantations. (Food and Agriculture Organization of the United Nations (FAO): Rome)
Mhlongo MI, Piater LA, Madala NE, Labuschagne N, Dubery IA (2018) The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front Plant Sci 9
Miller B, Madilao LL, Ralph S, Bohlmann J (2005) Insect-induced conifer defense. White pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol 137:369–382
Miller A, O'Reilly-Wapstra J, Potts B (2014) Genetic variation in bark stripping among Pinus radiata. National Centre for Future Forest Industries, Hobart.
Moran GF, Bell JC (1987) The origin and genetic diversity of Pinus radiata in Australia. Theor Appl Genet 73:616–622
Moreira X, Zas R, Sampedro L (2012a) Differential allocation of constitutive and induced chemical defenses in pine tree juveniles: a test of the optimal defense theory. PLoS ONE 7:e34006
Moreira X, Zas R, Sampedro L (2012b) Genetic variation and phenotypic plasticity of nutrient reallocation and increased fine root production as putative tolerance mechanisms inducible by methyl jasmonate in pine trees. J Ecol 100:810–820
Moreira X, Lundborg L, Zas R, Carrillo-Gavilán A, Borg-Karlson A-K, Sampedro L (2013a) Inducibility of chemical defences by two chewing insect herbivores in pine trees is specific to targeted plant tissue, particular herbivore and defensive trait. Phytochemistry 94:113–122
Moreira X, Zas R, Sampedro L (2013b) Additive genetic variation in resistance traits of an exotic pine species: little evidence for constraints on evolution of resistance against native herbivores. Heredity 110:449–456
Moreira X, Mooney KA, Rasmann S, Petry WK, Carrillo-Gavilán A, Zas R, Sampedro L (2014) Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecol Lett 17:537–546
Morkunas I, Ratajczak L (2014) The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol Plant 36:1607–1619
Morris WF, Traw MB, Bergelson J (2006) On testing for a tradeoff between constitutive and induced resistance. Oikos 112:102–110
Nantongo JS, Potts BM, Fitzgerald H, Newman J, Elms S, Aurik D, Dungey H, O’Reilly-Wapstra JM (2020) Quantitative genetic variation in bark stripping of Pinus radiata. Forests 11:1356
Nantongo JS, Potts B, Rodemann T, Fitzgerald H, Davies N, O’Reilly-Wapstra J (2021) Developing near infrared spectroscopy models for predicting chemistry and responses to stress in Pinus radiata (D. Don). J Near Infrared Spectrosc 29:4
NIST (2014) NIST/EPA/NIH Mass Spectral Library. In: Mass Spectral Search Program. (U.S. Government National Institute for Standards and Technology)
Page DE, Close D, Beadle CL, Wardlaw TJ, Mohammed CL (2013) Seasonal dynamics in understorey abundance and carbohydrate concentration in relation to browsing and bark stripping of Tasmanian Pinus radiata plantations. For Ecol Manag 296:98–107
Pearse IS, Paul R, Ode PJ (2018) Variation in plant defense suppresses herbivore performance. Curr Biol 28:1981-1986.e2
Piper FI, Fajardo A, Hoch G (2017) Single-provenance mature conifers show higher non-structural carbohydrate storage and reduced growth in a drier location. Tree Physiol 37:1001–1010
R Core Team (2018) R: a language and environment for statistical computing. In: (R Foundation for Statistical Computing: Vienna, Austria)
Raffa KF, Mason CJ, Bonello P, Cook S, Erbilgin N, Keefover-Ring K, Klutsch JG, Villari C, Townsend PA (2017) Defense syndromes in lodgepole—whitebark pine ecosystems relate to degree of historical exposure to mountain pine beetles. Plant Cell Environ 40:1791–1806
Ralph SG, Yueh H et al (2006) Conifer defence against insects: microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ 29:1545–1570
Reglinski T, Taylor JT, Northcott GL, Ah Chee A, Spiers M, Wohlers M, Hill RA (2017) Biochemical responses associated with induced resistance to Colletotrichum acutatum in Pinus radiata seedlings treated with methyl jasmonate and Trichoderma spp. For Pathol 47:e12350
Roth M, Hussain A, Cale JA, Erbilgin N (2018) Successful colonization of lodgepole pine trees by mountain pine beetle increased monoterpene production and exhausted carbohydrate reserves. J Chem Ecol 44:209–214
Saeki Y, Tuda M, Crowley PH (2014) Allocation tradeoffs and life histories: a conceptual and graphical framework. Oikos 123:786–793
Saint-Andrieux C, Bonenfant C, Toïgo C, Basille M, Klein F (2009) Factors affecting beech Fagus sylvatica bark stripping by red deer Cervus elaphus in a mixed forest. Wildl Biol 15:187–197
Salem MZM, Ali HM, Basalah MO (2014) Essential oils from wood, bark, and needles of Pinus roxburghii Sarg. from Alexandria, Egypt: antibacterial and antioxidant activities. BioResources 9:7454–7466
Sampedro L (2014) Physiological trade-offs in the complexity of pine tree defensive chemistry. Tree Physiol 34:915–918
Sampedro L, Moreira X, Zas R (2011) Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability. J Ecol 99:818–827
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY (2011) Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 8:1–10
Schwachtje J, Baldwin IT (2008) Why does herbivore attack reconfigure primary metabolism? Plant Physiol 146:845–851
Senior JK, Potts BM, O’Reilly-Wapstra JM, Bissett A, Wooliver RC, Bailey JK, Glen M, Schweitzer JA (2018) Phylogenetic trait conservatism predicts patterns of plant-soil feedback. Ecosphere 9:e02409
Snyder MA (1992) Selective herbivory by Abert’s squirrel mediated by chemical variability in ponderosa pine. Ecology 73:1730–1741
Stolter C, Niemelä P, Ball JP, Julkunen-Tiitto R, Vanhatalo A, Danell K, Varvikko T, Ganzhorn JU (2009) Comparison of plant secondary metabolites and digestibility of three different boreal coniferous trees. Basic Appl Ecol 10:19–26
Swett CL, Gordon TR (2017) Exposure to a pine pathogen enhances growth and disease resistance in Pinus radiata seedlings. For Pathol 47:e12298
Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci 5:293–293
Tomlin ES, Antonejevic E, Alfaro RI, Borden JH (2000) Changes in volatile terpene and diterpene resin acid composition of resistant and susceptible white spruce leaders exposed to simulated white pine weevil damage. Tree Physiol 20:1087–1095
Tsunoda T, van Dam NM (2017) Root chemical traits and their roles in belowground biotic interactions. Pedobiologia 65:58–67
Villari C, Faccoli M, Battisti A, Bonello P, Marini L (2014) Testing phenotypic trade-offs in the chemical defence strategy of Scots pine under growth-limiting field conditions. Tree Physiol 34:919–930
Welch D, Staines BW, Scott D, Catt DC (1988) Bark stripping damage by red deer in a Sitka spruce forest in western Scotland II. Wound Size and Position. Forestry 61:245–254
Wiley E, Helliker B (2012) A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol 195:285–289
Wink M (2008) Plant secondary metabolism: diversity, function and its evolution. Nat Product Commun 3, 1934578X0800300801
Zas R, Björklund N, Nordlander G, Cendán C, Hellqvist C, Sampedro L (2014) Exploiting jasmonate-induced responses for field protection of conifer seedlings against a major forest pest, Hylobius abietis. For Ecol Manag 313:212–223
Zhang S, Jiang J, Luan Q (2016) Genetic and correlation analysis of oleoresin chemical components in slash pine. Genet Mol Res 15, gmr.15038982
Zhang X, States JS (1991) Selective herbivory of ponderosa pine by Abert squirrels: a re-examination of the role of terpenes. Biochem Syst Ecol 19:111–115
Zhou S, Lou Y-R, Tzin V, Jander G (2015) Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol 169:1488–1498
Acknowledgements
We thank industrial partners, Timberlands Pacific Pty Ltd and the Radiata Pine Breeding company for the provision of genetic material. We also thank Paul Tilyard for assistance with sample collection and Dr David Nichols for the accurate mass data for molecular formula assignment. Judith Ssali Nantongo also acknowledges receipt of a Tasmania Graduate Research Scholarship.
Funding
Funding for this project was under Australian Research Council (ARC) Linkage Grant LP140100602.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Code availability
Not applicable.
Additional information
Communicated by Wolfgang Bilger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nantongo, J.S., Potts, B.M., Davies, N.W. et al. Variation in constitutive and induced chemistry in the needles, bark and roots of young Pinus radiata trees. Trees 36, 341–359 (2022). https://doi.org/10.1007/s00468-021-02209-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-021-02209-5