Abstract
Background
Tolvaptan preserves kidney function in adults with autosomal dominant polycystic kidney disease (ADPKD) at elevated risk of rapid progression. A trial (NCT02964273) evaluated tolvaptan safety and pharmacodynamics in children (5–17 years). However, progression risk was not part of study eligibility criteria due to lack of validated criteria for risk assessment in children. As risk estimation is important to guide clinical management, baseline characteristics of the study participants were retrospectively evaluated to determine whether risk of rapid disease progression in pediatric ADPKD can be assessed and to identify parameters relevant for risk estimation.
Methods
Four academic pediatric nephrologists reviewed baseline data and rated participant risk from 1 (lowest) to 5 (highest) based on clinical judgement and the literature. Three primary reviewers independently scored all cases, with each case reviewed by two primary reviewers. For cases with discordant ratings (≥ 2-point difference), the fourth reviewer provided a secondary rating blinded to the primary evaluations. Study participants with discordant ratings and/or for whom data were lacking were later discussed to clarify parameters relevant to risk estimation.
Results
Of 90 evaluable subjects, primary reviews of 69 (77%) were concordant. The proportion considered at risk of rapid progression (final mean rating ≥ 3.5) by age group was: 15–17 years, 27/34 (79%); 12– < 15, 9/32 (28%); 4– < 12, 8/24 (33%). The panelists agreed on characteristics important for risk determination: age, kidney imaging, kidney function, blood pressure, urine protein, and genetics.
Conclusions
High ratings concordance and agreement among reviewers on relevant clinical characteristics support the feasibility of pediatric risk assessment.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) has historically been considered as an adult disease [1]. Rates of cyst progression leading to chronic kidney disease are highly variable, however, with approximately 3% of children who carry ADPKD-causing mutations experiencing either early-onset or unusually progressive disease [2]. Moreover, there is an increasing awareness that, even in patients who are asymptomatic during childhood, the disease manifests in detectable ways. Cyst formation and expansion initiate early in life and affect kidney function before any decline in glomerular filtration rate (GFR) occurs [3]. Reduction in urinary concentrating capacity is one of the earliest signs of ADPKD observed in children and is present in 58% of the affected pediatric population [4,5,6]. Twenty percent of pediatric patients with ADPKD exhibit proteinuria, and glomerular hyperfiltration has been described in 18–21% [6, 7]. Disease progression may be reflected in extra-renal complications, with a reported hypertension prevalence of 20% in the affected pediatric population versus 2% in this age group among unaffected individuals. Although symptoms are less common in children and adolescents than adults, some pediatric patients report cyst-related pain [6]. In general, symptoms and psychological impacts of early-stage ADPKD are underestimated by clinicians [8].
Given that ADPKD is now understood as a longitudinal disease starting in childhood, disease-modifying treatment may exert the greatest benefit if initiated before most of the intact kidney parenchyma has been lost, while kidney function is maintained. Time to kidney failure would thus be delayed as compared to later intervention.
Tolvaptan is available for the treatment of ADPKD to delay decline in kidney function in adults (≥ 18 years) at risk of rapid progression [9]. A clinical trial (EudraCT number: 2016–000187-42; ClinicalTrials.gov identifier NCT02964273) evaluated the safety and pharmacodynamics of tolvaptan in children and adolescents with ADPKD [10, 11]. The study protocol and the first results of the randomized, double-blind phase have been reported [10, 11]. As validated diagnostic criteria for childhood ADPKD are lacking [1, 12], the presence of ADPKD was determined in the study subjects using criteria determined by expert consensus [10].
A possible extension of the adult indication to pediatric patients presupposes that children at risk of rapid progression can be identified. Tools exist for the identification of adult patients at elevated risk of rapid progression but cannot be applied to the pediatric population. The ADPKD risk classification algorithm developed by Mayo Clinic researchers is validated only for patients ages 15 years and over [13]. The Predicting Renal Outcomes in ADPKD (PROPKD) score cannot be calculated for patients age < 35 years in the absence of hypertension and onset of first urologic event [14]. Genetic assessment alone cannot be used to determine risk of rapid progression. Although ADPKD is known to be caused by mutations in the PKD1 or PKD2 genes, the genotype–phenotype relationship is complex, with a high amount of allelic heterogeneity [15]. In the absence of a validated risk stratification system, the tolvaptan pediatric study enrolled a broad population of subjects diagnosed with ADPKD, and risk of rapid progression was not part of the eligibility criteria [10]. To determine whether risk of rapid disease progression in pediatric ADPKD can be assessed and to identify parameters most relevant to risk estimation in this population, an expert panel was convened to retrospectively evaluate the baseline characteristics of patients enrolled in the tolvaptan pediatric study.
Methods
Design
As reported, key enrollment criteria for the tolvaptan pediatric study were ages 4 to 17 years (inclusive) and a diagnosis of ADPKD [10]. Diagnosis was based on family history and/or genetic evidence in conjunction with the confirmed presence of kidney cysts during study screening. Specifically, subjects aged 12–17 were required to have ≥ 10 kidney cysts on MRI, each cyst measuring ≥ 0.5 cm. Those aged 4–11, who did not undergo MRI, were required to have ≥ 4 kidney cysts on ultrasound, each ≥ 1 cm. Subjects also had to have eGFR ≥ 60 mL/min/1.73 m2 by the bedside Schwartz formula [16].
Assessments performed at study screening and/or baseline visits included vital signs, serum chemistry, urinalysis, medical history, MRI/ultrasound imaging, and if available, ADPKD genetic results. The screening and baseline visits were to be made within 31 days of each other. For the present analysis, subject data from the screening/baseline assessments were retrospectively evaluated.
The study sponsor convened a panel of four expert pediatric nephrologists (MC, SG, DM, LGW) to review the subject data and determine the risk of rapid progression for each subject. Data on each of the 91 enrolled subjects was extracted from the study database and placed into a separate document for each subject for distribution to panel members using a secure data transfer portal. Initially, a pilot review was conducted in which the patient profiles of six study subjects were distributed to three of the reviewers to test the distribution process and assess review parameters. The reviewers were asked to review each subject on a 5-point scale, with a score of 1 denoting that the subject is “least likely to be a rapid progressor” and 5 denoting that the subject is “most likely to be a rapid progressor”. Reviewers were also asked to consider the feasibility of the 5-point scale, how to describe the level of risk that should be assigned to each score (e.g., 1 – unlikely, 2 – possible, 3 – probable, etc.), and to describe the rationale for the ratings assigned to each subject. A meeting with the reviewers was subsequently held to compare the ratings assigned by each reviewer and to discuss the appropriateness of the process and parameters used. This pilot also dismissed the need to consider a 7-point scale, as all reviewers unanimously agreed to the sufficiency of the 5-point scale for pilot cases.
Following feasibility as determined in the pilot review, review and scoring of patient profiles for all enrolled subjects was conducted. There were three primary reviewers, and the fourth reviewer provided additional review for discordant cases. Each of three primary reviewers received a portion of the 91 profiles (60–61 profiles for each reviewer), so that each profile would be independently reviewed and scored by two primary reviewers in blinded fashion. The ratings assigned by each of the two primary reviewers were compared by the study sponsor. For study subjects for whom the assigned ratings were discordant (defined as a difference of ≥ 2 points between reviewers), the fourth panel member would provide an additional, secondary review and rating while blinded to the primary evaluations. The results of this additional review were recorded separately. This same panel member would also provide a blinded, independent rating for cases in which one of the primary reviewers believed there would be value to having another expert review of any subject and requested such additional input.
Finally, a panel meeting of the expert reviewers and study sponsor was held to discuss the ratings and compare the scores given by different reviewers, identify the key characteristics of patients at risk for rapid progression, and determine the final individual reviewer ratings.
Outcomes
The number and percentage of concordant ratings assigned by the blinded primary reviewers were determined and interrater agreement was statistically evaluated as described below. Another outcome of interest was the key parameters for assessing risk of rapid progression that were identified by the expert panelists during the discussion. Potential key parameters considered for estimating risk of rapid progression were based on the literature and the panelists’ clinical experience (Table 1) [6, 13, 15, 17,18,19,20,21].
For subjects who were assigned discordant ratings and/or had their ratings adjusted during the group discussion due to inadequate or missing data, the participants explored strategies for risk assessment when only limited information is available. Examples are presented to illustrate the factors considered by the panelists to be most important in assessing such cases.
Statistical analyses
Interrater agreement among the primary reviewers was assessed using the Bland–Altman method [34]. Final overall ratings were determined by weighting the rating of each primary rater equally, and in cases where an additional, secondary review was provided by the fourth panel member, the secondary rating was weighted at 1.5 to provide a clear tie-breaker: [(1x + 1x)/2 OR (1x + 1x + 1.5x)/3.5)].
Results
Analysis population
As reported, 91 subjects with ADPKD aged 5–17 years were enrolled. In the overall study population and within age subgroups (12–17 years, 4–11 years), study subjects exhibited wide ranges for height-adjusted total kidney volume and estimated glomerular filtration rate, suggesting a broad spectrum of ADPKD severity among the enrolled subjects (Table 2). Diagnostic evaluation included genetic testing in 27/91 (30%) of subjects.
Pilot evaluation
The process and parameters used were confirmed to be appropriate, and the team agreed to proceed with a full patient profile review of all 91 subjects and use the 5-point scale.
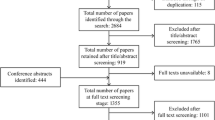
Full study population review
From a total of 91 subjects with ADPKD, 22 required an additional review by an independent panelist due to discordant primary ratings (≥ 2-point difference; n = 12), one of the primary raters not providing an initial rating due to questions about the data provided (n = 7), or the reviewers recommending subjects for exclusion from review (n = 3) because of missing kidney volume data at screening and baseline (Fig. 1). During the panel discussion, postbaseline kidney volume data were provided for the 3 subjects recommended for exclusion due to missing screening and baseline kidney volumes. The expert reviewers agreed that the 3 subjects could be assigned ratings for risk of progression based on the additional data, and all raters considered these 3 subjects to have large kidney volumes and to be at high risk of rapid progression. All panelists agreed that another of the 22 subjects requiring additional review should be excluded from risk assessment due to a history of suspected ureteropelvic junction obstruction, yielding a consensus that 90 of the 91 subjects were evaluable for progression risk.
Results of ratings by the 2 initial reviewers. *These subjects were later, during the panel discussion, deemed evaluable for progression risk based on additional kidney volume data provided at the meeting and were assigned ratings for progression risk at that time. Because reviewers initially recommended exclusion of these 3 subjects from risk assessment, they are excluded from the analysis of interrater agreement
Of the 90 subjects deemed evaluable, panelist reviews of 69 cases (77%) were concordant (≤ 1-point difference) in the first round and did not need an additional independent and blinded review. Individual reviewer ratings were adjusted for 11 subjects during the discussion to assign final ratings, including subjects for whom the primary ratings were and were not concordant. Reasons for the rating changes were based on missing or misunderstood patient data or data entry issues in assigning the ratings, including transcription errors by the reviewer, listing more than one rating score by reviewer (e.g., “a 4 or a 5”), and not providing a rating due to questions about the data (these were resolved by answering the questions and the reviewer providing a rating).
Results of the Bland–Altman analysis to assess interrater agreement among the blinded primary reviewers for the 87 subjects who were included in the primary review are shown in Fig. 2. This group consisted of the 91 total subjects less the 3 initially recommended for exclusion from the analysis due to missing data and the 1 deemed not evaluable by the panel because of suspected ureteropelvic obstruction. The mean of the two ratings for a subject is plotted on the x-axis and the difference between the two ratings is plotted on the y-axis (the plots do not distinguish identical scores). The middle line is the mean difference between ratings for all subjects (0.356 points) with a 95% confidence interval (CI). Using standard limits of agreement (i.e., ± 1.96 × standard deviation of observed differences) [34], indicated by the upper and lower lines (with 95% CI), only 2 points exceeded the 95% CI of the upper limit, and the other points were all within the limits of agreement. Thus, there was minimal evidence that scores of the primary raters differed significantly.
The number and percentage of subjects considered at risk of rapid progression (final weighted mean score of at least 3.5) by age group was: 15 to 17 years, 27/34 (79%); 12 to < 15 years, 9/32 (28%); 4 to < 12 years, 8/24 (33%). Twenty-five of 34 (74%) subjects ≥ 15 to 17 years of age were classified as Class 1C–1E using adult ADPKD risk classification (i.e., Mayo classification), which can be applied in that age group [13]. In that age group, an additional 2 subjects were judged to be at risk of rapid progression in the present study due to eGFR status despite being assigned to Class 1A and 1B.
In addition to genetic data, the panelists reached agreement on key phenotypic characteristics relevant to risk determination (in no particular order of importance): kidney imaging including volume, kidney function, blood pressure, and urine protein, with earlier age at onset and/or worsening of each factor indicating greater ADPKD progression risk. Several cases in which data were lacking or ambiguous and required panelist discussion about how to best assess progression risk are provided in Online Resource 1 to illustrate how the panelists resolved cases for whom incomplete information was available.
Discussion
In this retrospective assessment for risk of rapid progression in pediatric patients with ADPKD, an expert review panel produced concordant initial risk ratings for 77% of the study participants, with a strong level of interrater agreement. This result supports that a subpopulation of children with ADPKD who are at high risk of rapid progression can be identified based on previously reported adult and/or pediatric risk factors and panel expertise. Even for cases in which all the desired information for risk assessment was not available, the reviewers were able to reach agreement on the risk level and which variables were most important. For both adults and children with ADPKD, it is common to encounter situations in the real-world clinical setting in which prognostic data are lacking [35].
It is reassuring that for the 15–17 age group, in this exercise 27/34 (79%) subjects were considered at risk of rapid progression, which is similar to the 25/34 (74%) that would be classified as high risk based on total kidney volume risk classification for patients with ADPKD aged 15 years and older [13]. The smaller proportions of participants in the younger age groups who were categorized as high risk (28% for ages 12 to < 15 years and 33% for ages 4 to < 12 years) may not reflect the true percentages of high-risk participants in those age strata; risk of progression may be more difficult to determine at younger ages with fewer or less advanced clinical disease manifestations. Even with this caveat, a notable finding was that approximately one-third of subjects aged 4 to < 15 years were evaluated as at high risk of rapid progression. In the youngest cohorts of individuals with ADPKD, a substantial proportion already has evidence of aggressive disease and will require more intensive clinical management in the opinion of reviewers and based on the data provided. Accordingly, in pediatric nephrology practice, individual disease phenotype should be considered more important than patient age.
The choice of age groups for categorization in this analysis (4 to < 12 years, 12 to < 15 years, and 15 to 17 years) was based on the age-appropriate imaging methodology for assessing kidney volume in the trial (i.e., MRI in subjects aged 12 to 17 years and ultrasound in those aged 4 to < 12 years) as well as the 3 age categories used in the evaluation of tolvaptan for pediatric use by regulatory authorities.
The panelists agreed that key characteristics relevant to risk determination were: kidney imaging, kidney function, blood pressure, urine protein, and genetics, with earlier age of onset and/or worsening of the clinical manifestations indicating higher ADPKD progression risk. These markers are qualitatively consistent with diagnostic criteria for adults [13, 14, 36]. As treatment for pediatric ADPKD evolves, including the potential use of disease-specific therapy in this population, accepted criteria for risk assessment will likely be needed to guide insurance reimbursement decisions.
Assigning weights to the respective progression risk factors identified in this study and developing a formal risk scoring system will require large-scale, longitudinal, and comprehensive assessment of pediatric ADPKD populations. Even if evaluation for risk of progression is systematized, the endpoints chosen for outcome assessment in ADPKD have been inconsistent across studies conducted in adults as well as in children and will need to be harmonized in the future [37]. At present, kidney volumetric measurement is the most promising method for accurate risk assessment. Three-dimensional ultrasound imaging methods suitable for children and a pediatric risk classification system adapted from Mayo Imaging Classification were recently shown to be feasible, although they require validation [17]. Further, more research on normal pediatric kidney dimensions, volume, and growth is needed to understand kidney growth in pediatric ADPKD [17, 22]. The presence of hypertension and urine protein are good indicators of progression risk and should be monitored [22, 38]. Decreased kidney function is a strong indicator of rapidly progressive disease, but in most pediatric patients, function is preserved, which limits the predictive utility of this risk factor [38]. Further, the applicability of the most commonly used equation for estimating eGFR in children (CKiD) has been questioned for the ADPKD population [39]. Although PKD1 truncating and nontruncating variants are known to be associated with greater risk of rapidly progressive ADPKD, genetic determinants are too complex to accurately predict disease course [22]. Risk prediction is likely to be enhanced by biomarkers, some of which have demonstrated predictive value in adult ADPKD (plasma copeptin, urinary monocyte chemoattractant protein-1), and which require research in children [19, 20, 36, 38]. Kidney cyst quantification or textural analysis to detect structural changes in cystic or noncystic tissue, enhanced by artificial intelligence/machine learning methods, may enhance the prognostic value of imaging [36, 38].
Limitations of this research include that the data analyzed were obtained from a study of participants under the care of academic pediatric nephrology centers and therefore likely to be more serious cases and not representative of the ADPKD population as a whole. Further, the retrospective analysis reported here was not designed to generate a formalized, validated assessment system, but to demonstrate that children with ADPKD who are at increased risk of rapid progression can be identified based on clinical judgment and to specify the most relevant parameters for determining increased risk. Given the continuing challenges to diagnosis, prognostic assessment, and treatment decision-making in ADPKD, the present research advances the understanding of this condition in the affected pediatric population despite caveats to the findings.
The results of this analysis indicate that assessment of risk for rapid progression is feasible in pediatric patients with ADPKD and that the key parameters for risk assessment identified by expert consensus are consistent with those used in adult risk assessment. As recognition of ADPKD as a disease affecting the pediatric population increases, differentiated assessment of individual phenotype, regardless of patient age, will assume greater importance in clinical management.
Data availability
To submit inquiries related to Otsuka clinical research, or to request access to individual participant data (IPD) associated with any Otsuka clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data sharing platform.
References
De Rechter S, Breysem L, Mekahli D (2017) Is autosomal dominant polycystic kidney disease becoming a pediatric disorder? Front Pediatr 5:272. https://doi.org/10.3389/fped.2017.00272
Gimpel C, Bergmann C, Bockenhauer D et al (2019) International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat Rev Nephrol 15:713–726. https://doi.org/10.1038/s41581-019-0155-2
Helal I, Reed B, McFann K, Yan XD, Fick-Brosnahan GM, Cadnapaphornchai M, Schrier RW (2011) Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6:2439–2443. https://doi.org/10.2215/cjn.01010211
Fick GM, Duley IT, Johnson AM, Strain JD, Manco-Johnson ML, Gabow PA (1994) The spectrum of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol 4:1654–1660. https://doi.org/10.1681/asn.v491654
Ho TA, Godefroid N, Gruzon D, Haymann JP, Maréchal C, Wang X, Serra A, Pirson Y, Devuyst O (2012) Autosomal dominant polycystic kidney disease is associated with central and nephrogenic defects in osmoregulation. Kidney Int 82:1121–1129. https://doi.org/10.1038/ki.2012.225
De Rechter S, Bockenhauer D, Guay-Woodford LM et al; for the ADPedKD Consortium (2019) ADPedKD: a global online platform on the management of children with ADPKD. Kidney Int Rep 4:1271–1284. https://doi.org/10.1016/j.ekir.2019.05.015
Marlais M, Cuthell O, Langan D, Dudley J, Sinha MD, Winyard PJ (2016) Hypertension in autosomal dominant polycystic kidney disease: a meta-analysis. Arch Dis Child 101:1142–1147. https://doi.org/10.1136/archdischild-2015-310221
Baker A, King D, Marsh J, Makin A, Carr A, Davis C, Kirby C (2015) Understanding the physical and emotional impact of early-stage ADPKD: experiences and perspectives of patients and physicians. Clin Kidney J 8:531–537. https://doi.org/10.1093/ckj/sfv060
JYNARQUE (2020) US prescribing information. Otsuka Pharmaceutical Co., Ltd., Tokyo. https://www.otsuka-us.com/sites/g/files/qhldwo6171/files/media/static/JYNARQUE-PI.pdf. Accessed 4 Jun 2023
Schaefer F, Mekahli D, Emma F, Gilbert RD, Bockenhauer D, Cadnapaphornchai MA, Shi L, Dandurand A, Sikes K, Shoaf SE (2019) Tolvaptan use in children and adolescents with autosomal dominant polycystic kidney disease: rationale and design of a two-part, randomized, double-blind, placebo-controlled trial. Eur J Pediatr 178:1013–1021. https://doi.org/10.1007/s00431-019-03384-x
Mekahli D, Guay-Woodford L, Cadnapaphornchai MA, Greenbaum LA, Litwin M, Seeman T, Dandurand A, Shi L, Sikes K, Shoaf SE, Schaefer F (2023) Tolvaptan for children and adolescents with autosomal dominant polycystic kidney disease: randomized controlled trial. Clin J Am Soc Nephrol 18:36–46. https://doi.org/10.2215/CJN.0000000000000022
Gimpel C, Bergmann C, Mekahli D (2022) The wind of change in the management of autosomal dominant polycystic kidney disease in childhood. Pediatr Nephrol 37:473–487. https://doi.org/10.1007/s00467-021-04974-4
Irazabal MV, Rangel LJ, Bergstralh EJ et al; CRISP Investigators (2015) Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 26:160–172.https://doi.org/10.1681/asn.2013101138
Cornec-Le Gall E, Audrézet MP, Rousseau A et al (2016) The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27:942–951. https://doi.org/10.1681/asn.2015010016
Cornec-Le Gall E, Audrézet MP, Chen JM et al (2013) Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24:1006–1013. https://doi.org/10.1681/asn.2012070650
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. https://doi.org/10.1681/asn.2008030287
Breysem L, De Keyzer F, Schellekens P, Dachy A, De Rechter S, Janssens P, Vennekens R, Bammens B, Irazabal MV, Van Ongeval C, Harris PC, Mekahli D; CRISP Consortium (2023) Risk severity model for pediatric autosomal dominant polycystic kidney disease using 3D ultrasound volumetry. Clin J Am Soc Nephrol 18:581–591.https://doi.org/10.2215/cjn.0000000000000122
Nowak KL, You Z, Gitomer B, Brosnahan G, Torres VE, Chapman AB, Perrone RD, Steinman TI, Abebe KZ, Rahbari-Oskoui FF, Yu ASL, Harris PC, Bae KT, Hogan M, Miskulin D, Chonchol M (2018) Overweight and obesity Are predictors of progression in early autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29:571–578. https://doi.org/10.1681/asn.2017070819
Zheng D, Wolfe M, Cowley BD Jr, Wallace DP, Yamaguchi T, Grantham JJ (2003) Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14:2588–2595. https://doi.org/10.1097/01.asn.0000088720.61783.19
Messchendorp AL, Meijer E, Visser FW, Engels GE, Kappert P, Losekoot M, Peters DJM, Gansevoort RT; on behalf of the DIPAK-1 study investigators (2019) Rapid progression of autosomal dominant polycystic kidney disease: urinary biomarkers as predictors. Am J Nephrol 50:375-385. https://doi.org/10.1159/000502999
Azukaitis K, Ju W, Kirchner M et al; ESCAPE Trial Group (2019) Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int 96:214–221. https://doi.org/10.1016/j.kint.2019.01.035
Dachy A, Van Loo L, Mekahli D (2023) Autosomal dominant polycystic kidney disease in children and adolescents: assessing and managing risk of progression. Adv Kidney Dis Health 30:236–244. https://doi.org/10.1053/j.akdh.2023.01.007
Mekahli D, Woolf AS, Bockenhauer D (2010) Similar renal outcomes in children with ADPKD diagnosed by screening or presenting with symptoms. Pediatr Nephrol 25:2275–2282. https://doi.org/10.1007/s00467-010-1617-8
Selistre L, de Souza V, Ranchin B, Hadj-Aissa A, Cochat P, Dubourg L (2012) Early renal abnormalities in children with postnatally diagnosed autosomal dominant polycystic kidney disease. Pediatr Nephrol 27:1589–1593. https://doi.org/10.1007/s00467-012-2192-y
Wong H, Vivian L, Weiler G, Filler G (2004) Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am J Kidney Dis 43:624–628. https://doi.org/10.1053/j.ajkd.2003.12.026
Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH (1992) Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41:1311–1319. https://doi.org/10.1038/ki.1992.195
Fick-Brosnahan GM, Tran ZV, Johnson AM, Strain JD, Gabow PA (2001) Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int 59:1654–1662. https://doi.org/10.1046/j.1523-1755.2001.0590051654.x
Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW (2011) Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 6:369–376. https://doi.org/10.2215/cjn.03780410
Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL (1995) Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 123:754–762. https://doi.org/10.7326/0003-4819-123-10-199511150-00003
Ruggenenti P, Perna A, Mosconi L, Matalone M, Pisoni R, Gaspari F, Remuzzi G (1997) Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 63:S54–S57
Gansevoort RT, Meijer E, Chapman AB, Czerwiec FS, Devuyst O, Grantham JJ, Higashihara E, Krasa HB, Ouyang J, Perrone RD, Torres VE; TEMPO 3:4 Investigators, (2016) Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 Trial. Nephrol Dial Transplant 31:1887–1894. https://doi.org/10.1093/ndt/gfv422
Dicks E, Ravani P, Langman D, Davidson WS, Pei Y, Parfrey PS (2006) Incident renal events and risk factors in autosomal dominant polycystic kidney disease: a population and family-based cohort followed for 22 years. Clin J Am Soc Nephrol 1:710–717. https://doi.org/10.2215/cjn.01581105
Fencl F, Janda J, Bláhová K, Hríbal Z, Stekrová J, Puchmajerová A, Seeman T (2009) Genotype-phenotype correlation in children with autosomal dominant polycystic kidney disease. Pediatr Nephrol 24:983–989. https://doi.org/10.1007/s00467-008-1090-9
Ranganathan P, Pramesh CS, Aggarwal R (2017) Common pitfalls in statistical analysis: measures of agreement. Perspect Clin Res 8:187–191. https://doi.org/10.4103/picr.picr_123_17
Grantham JJ, Torres VE (2016) The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 12:667–677. https://doi.org/10.1038/nrneph.2016.135
Chebib FT, Torres VE (2021) Assessing risk of rapid progression in autosomal dominant polycystic kidney disease and special considerations for disease-modifying therapy. Am J Kidney Dis 78:282–292. https://doi.org/10.1038/nrneph.2016.135
Jdiaa SS, Husainat NM, Mansour R, Kalot MA, McGreal K, Chebib FT, Perrone RD, Yu A, Mustafa RA (2022) A systematic review of reported outcomes in ADPKD studies. Kidney Int Rep 7:1964–1979. https://doi.org/10.1016/j.ekir.2022.06.012
Mekahli D, Womack H, Dahl NK (2022) Perspectives on drug development in early ADPKD. Clin J Am Soc Nephrol 17:1555–1558. https://doi.org/10.2215/cjn.05190422
Schellekens P, Verjans M, Janssens P, Dachy A, De Rechter S, Breysem L, Allegaert K, Bammens B, Vennekens R, Vermeersch P, Pottel H, Mekahli D (2023) Low agreement between various eGFR formulae in pediatric and young adult ADPKD patients. Pediatr Nephrol 38:3043–3053. https://doi.org/10.1007/s00467-023-05926-w
Acknowledgements
This work was funded by Otsuka Pharmaceutical Development & Commercialization, Inc (Princeton, NJ, USA). Writing and editorial services in preparation of the manuscript, including the drafting of the first version of the manuscript, were provided by BioScience Communications, Inc (New York, NY, USA), activities that were also funded by Otsuka.
Funding
This work was funded by Otsuka Pharmaceutical Development & Commercialization, Inc (Princeton, NJ, USA).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SLG. All authors commented on and provided substantive intellectual input into each version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
As reported previously (Schaefer F et al., Eur J Pediatr 2019;178:1013–1021), the clinical trial from which the data analyzed here were obtained was conducted in conformity with FDA regulations, International Conference on Harmonization Good Clinical Practice Guideline (E6), international ethical principles derived from the Declaration of Helsinki, Council for International Organizations of Medical Science guidelines, and applicable local laws and regulations.
Informed consent
As reported previously for the original clinical trial from which the data analyzed here were obtained (Schaefer F et al., Eur J Pediatr 2019;178:1013–1021), written informed consent was obtained from a parent/guardian or legally acceptable representative, as applicable for local laws, at screening, prior to the initiation of any protocol-required procedures. In addition, the subject must provide age-appropriate informed assent at screening and must have been able to understand that he or she could withdraw from the trial at any time.
Competing interests
Dr. Mekahli, as a representative of UZ Leuven and KU Leuven, reports research grants from Otsuka Pharmaceuticals, and serves on advisory boards for Otsuka Pharmaceuticals, Sanofi Genzyme, and Reata, all paid to her institutions UZ Leuven and KU Leuven. She is supported by the Research Foundation Flanders (FWO) (G0C8920N and G060623N) and clinical senior research grant for D. Mekahli (1804123N).
Dr. Guay-Woodford serves a consultant for and receives honoraria from Otsuka and Natera, Inc.
Dr. Cadnapaphornchai serves on a pediatric advisory board of Otsuka Pharmaceuticals Inc.
Dr. Goldstein has received grant funding from and served as a consultant for Otsuka.
Dr. Dandurand was formerly employed by Otsuka.
Dr. Jiang is an employee of Otsuka.
Dr. Jadhav was formerly employed by Otsuka and serves as a consultant for Otsuka.
Ms. Debuque is an employee of Otsuka.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mekahli, D., Guay-Woodford, L.M., Cadnapaphornchai, M.A. et al. Estimating risk of rapid disease progression in pediatric patients with autosomal dominant polycystic kidney disease: a randomized trial of tolvaptan. Pediatr Nephrol 39, 1481–1490 (2024). https://doi.org/10.1007/s00467-023-06239-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06239-8