Abstract
Background
Children with persistent, isolated microscopic hematuria typically undergo a limited diagnostic workup and are monitored for signs of kidney disease in long-term longitudinal follow-up, which can delay diagnosis and allow disease progression in some cases.
Methods
To determine the clinical utility of genetic screening in this population, we performed targeted genetic testing using a custom, 32-gene next-generation sequencing panel for progressive kidney disease on children referred to the Texas Children’s Hospital Pediatric Nephrology clinic for persistent, microscopic hematuria (n = 30; cohort 1). Patients with microscopic hematuria identified by urinalysis on at least two separate occasions were eligible for enrollment, but those with other evidence of kidney disease were excluded. Results were analyzed for sequence variants using the American College of Medical Genetics and Genomics (ACMG) guideline for data interpretation and were validated using a secondary analysis of a dataset of children with hematuria and normal kidney function who had undergone genetic testing as part of an industry-sponsored program (cohort 2; n = 67).
Results
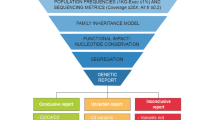
In cohort 1 33% of subjects (10/30) had pathogenic or likely pathogenic (P/LP) variants in the type IV collagen genes (COL4A3/A4/A5), and 10% (3/30) had variants of uncertain significance in these genes. The high diagnostic rate in type IV collagen genes was confirmed in cohort 2, where 27% (18/67) of subjects had P/LP variants in COL4A3/A4/A5 genes.
Conclusions
Children with persistent, isolated microscopic hematuria have a high likelihood of having pathogenic variants in type IV collagen genes and genetic screening should be considered.
Graphical Abstract

A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
References
Vehaskari VM, Rapola J, Koskimies O, Savilahti E, Vilska J, Hallman N (1979) Microscopic hematuria in school children: epidemiology and clinicopathologic evaluation. J Pediatr 95:676–684
Vivante A, Afek A, Frenkel-Nir Y, Tzur D, Farfel A, Golan E, Chaiter Y, Shohat T, Skorecki K, Calderon-Margalit R (2011) Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA 306:729–736. https://doi.org/10.1001/jama.2011.1141
Moreno JA, Yuste C, Gutiérrez E, Sevillano ÁM, Rubio-Navarro A, Amaro-Villalobos JM, Praga M, Egido J (2016) Haematuria as a risk factor for chronic kidney disease progression in glomerular diseases: a review. Pediatr Nephrol 31:523–533. https://doi.org/10.1007/s00467-015-3119-1
Túri S, Visy M, Vissy A, Jászai V, Czirbesz Z, Haszon I, Szelid Z, Ferkis I (1989) Long-term follow-up of patients with persistent/recurrent, isolated haematuria: a Hungarian multicentre study. Pediatr Nephrol 3:235–239. https://doi.org/10.1007/BF00858521
Feng CY, Xia YH, Wang WJ, Xia J, Fu HD, Wang X, Shen HJ, Qian GL, Liu AM, Mao JH (2013) Persistent asymptomatic isolated hematuria in children: clinical and histopathological features and prognosis. World J Pediatr 9:163–168. https://doi.org/10.1007/s12519-013-0415-3
Hoshino Y, Kaga T, Abe Y, Endo M, Wakai S, Tsuchiya K, Nitta K (2015) Renal biopsy findings and clinical indicators of patients with hematuria without overt proteinuria. Clin Exp Nephrol 19:918–924. https://doi.org/10.1007/s10157-015-1090-6
Lee HM, Hyun JI, Min J-W, Lee K, Kim YK, Choi EJ, Song HC (2016) The natural course of biopsy-proven isolated microscopic hematuria: a single center experience of 350 patients. J Korean Med Sci 31:909–914. https://doi.org/10.3346/jkms.2016.31.6.909
Kim BS, Kim YK, Shin YS, Kim YO, Song HC, Kim YS, Choi EJ (2009) Natural history and renal pathology in patients with isolated microscopic hematuria. Korean J Intern Med 24:356–361. https://doi.org/10.3904/kjim.2009.24.4.356
Lieu TA, Grasmeder HM 3rd, Kaplan BS (1991) An approach to the evaluation and treatment of microscopic hematuria. Pediatr Clin North Am 38:579–592
Kallash M, Rheault MN (2020) Approach to persistent microscopic hematuria in children. Kidney 360 https://doi.org/10.34067/KID.0003222020
Tondel C, Vikse BE, Bostad L, Svarstad E (2012) Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol 7:1591–1597. https://doi.org/10.2215/CJN.02150212
Varnell CDJ, Stone HK, Welge JA (2019) Bleeding complications after pediatric kidney biopsy: a systematic review and meta-analysis. Clin J Am Soc Nephrol 14:57–65. https://doi.org/10.2215/CJN.05890518
Feld LG, Meyers KE, Kaplan BS, Stapleton FB (1998) Limited evaluation of microscopic hematuria in pediatrics. Pediatrics 102:E42
Bergstein J, Leiser J, Andreoli S (2005) The clinical significance of asymptomatic gross and microscopic hematuria in children. Arch Pediatr Adolesc Med 159:353–355. https://doi.org/10.1001/archpedi.159.4.353
Bullich G, Domingo-Gallego A, Vargas I, Ruiz P, Lorente-Grandoso L, Furlano M, Fraga G, Madrid Á, Ariceta G, Borregán M, Piñero-Fernández JA, Rodríguez-Peña L, Ballesta-Martínez MJ, Llano-Rivas I, Meñica MA, Ballarín J, Torrents D, Torra R, Ars E (2018) A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int 94:363–371. https://doi.org/10.1016/j.kint.2018.02.027
Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, Coppo R, Emma F, Johnson S, Karpman D, Landau D, Langman CB, Lapeyraque AL, Licht C, Nester C, Pecoraro C, Riedl M, van de Kar NC, Van de Walle J, Vivarelli M, Frémeaux-Bacchi V; HUS International (2015) An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. https://doi.org/10.1007/s00467-015-3076-8
Deltas C, Pierides A, Voskarides K (2013) Molecular genetics of familial hematuric diseases. Nephrol Dial Transplant 28:2946–2960. https://doi.org/10.1093/ndt/gft253
Tryggvason K, Patrakka J (2006) Thin basement membrane nephropathy. J Am Soc Nephrol 17:813–822. https://doi.org/10.1681/ASN.2005070737
Kashtan CE (2009) Familial hematuria. Pediatr Nephrol 24:1951–1958. https://doi.org/10.1007/s00467-007-0622-z
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR (2014) ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 42:D980-985. https://doi.org/10.1093/nar/gkt1113
Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, Massouras A (2019) VarSome: the human genomic variant search engine. Bioinformatics 35:1978–1980. https://doi.org/10.1093/bioinformatics/bty897
Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, den Dunnen JT (2011) LOVD vol 2.0: the next generation in gene variant databases. Hum Mutat 32:557–563. https://doi.org/10.1002/humu.21438
Savige J, Storey H, Watson E, Hertz JM, Deltas C, Renieri A, Mari F, Hilbert P, Plevova P, Byers P, Cerkauskaite A, Gregory M, Cerkauskiene R, Ljubanovic DG, Becherucci F, Errichiello C, Massella L, Aiello V, Lennon R, Hopkinson L, Koziell A, Lungu A, Rothe HM, Hoefele J, Zacchia M, Martic TN, Gupta A, van Eerde A, Gear S, Landini S, Palazzo V, Al-Rabadi L, Claes K, Corveleyn A, Van Hoof E, van Geel M, Williams M, Ashton E, Belge H, Ars E, Bierzynska A, Gangemi C, Lipska-Ziętkiewicz BS (2021) Consensus statement on standards and guidelines for the molecular diagnostics of Alport syndrome: refining ACMG criteria. Eur J Hum Genet 29:1186–1197. https://doi.org/10.1038/s41431-021-00858-1
Artuso R, Fallerini C, Dosa L, Scionti F, Clementi M, Garosi G, Massella L, Epistolato MC, Mancini R, Mari F, Longo I, Ariani F, Renieri A, Bruttini M (2012) Advances in Alport syndrome diagnosis using next-generation sequencing. Eur J Hum Genet 20:50–57. https://doi.org/10.1038/ejhg.2011.164
Papazachariou L, Demosthenous P, Pieri M, Papagregoriou G, Savva I, Stavrou C, Zavros M, Athanasiou Y, Ioannou K, Patsias C, Panagides A, Potamitis C, Demetriou K, Prikis M, Hadjigavriel M, Kkolou M, Loukaidou P, Pastelli A, Michael A, Lazarou A, Arsali M, Damianou L, Goutziamani I, Soloukides A, Yioukas L, Elia A, Zouvani I, Polycarpou P, Pierides A, Voskarides K, Deltas C (2014) Frequency of COL4A3/COL4A4 mutations amongst families segregating glomerular microscopic hematuria and evidence for activation of the unfolded protein response Focal and segmental glomerulosclerosis is a frequent development during ageing. PLoS One 9:e115015. https://doi.org/10.1371/journal.pone.0115015
Gibson J, Fieldhouse R, Chan MMY, Sadeghi-Alavijeh O, Burnett L, Izzi V, Persikov AV, Gale DP, Storey H, Savige J; Genomics England Research Consortium (2021) Prevalence estimates of predicted pathogenic COL4A3–COL4A5 variants in a population sequencing database and their implications for Alport syndrome. J Am Soc Nephrol 32:2273–2290. https://doi.org/10.1681/ASN.2020071065
Hicks J, Mierau G, Wartchow E, Eldin K (2012) Renal diseases associated with hematuria in children and adolescents: a brief tutorial. Ultrastruct Pathol 36:1–18. https://doi.org/10.3109/01913123.2011.620731
Savige J (2014) Alport syndrome: its effects on the glomerular filtration barrier and implications for future treatment. J Physiol 592:4013–4023. https://doi.org/10.1113/jphysiol.2014.274449
LeBleu VS, Macdonald B, Kalluri R (2007) Structure and function of basement membranes. Exp Biol Med (Maywood) 232:1121–1129. https://doi.org/10.3181/0703-MR-72
Khoshnoodi J, Cartailler JP, Alvares K, Veis A, Hudson BG (2006) Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem 281:38117–38121
LeBleu V, Sund M, Sugimoto H, Birrane G, Kanasaki K, Finan E, Miller CA, Gattone VH 2nd, McLaughlin H, Shield CF 3rd, Kalluri R (2010) Identification of the NC1 domain of {alpha}3 chain as critical for {alpha}3{alpha}4{alpha}5 type IV collagen network assembly. J Biol Chem 285:41874–41885. https://doi.org/10.1074/jbc.M110.149534
Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL (2009) Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 20:1471–1479. https://doi.org/10.1681/ASN.2008101086
Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG (1997) Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest 99:2470–2478. https://doi.org/10.1172/JCI119431
Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, Nakanishi K, Nozu K, Renieri A, Rheault M, Wang F, Gross O (2018) Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int 93:1045–1051. https://doi.org/10.1016/j.kint.2017.12.018
Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC (2003) X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol 14:2603–2610. https://doi.org/10.1097/01.asn.0000090034.71205.74
Bekheirnia MR, Reed B, Gregory MC, McFann K, Shamshirsaz AA, Masoumi A, Schrier RW (2010) Genotype-phenotype correlation in X-linked Alport syndrome. J Am Soc Nephrol 21:876–883. https://doi.org/10.1681/ASN.2009070784
Yamamura T, Nozu K, Fu XJ, Nozu Y, Ye MJ, Shono A, Yamanouchi S, Minamikawa S, Morisada N, Nakanishi K, Shima Y, Yoshikawa N, Ninchoji T, Morioka I, Kaito H, Iijima K (2017) Natural history and genotype-phenotype correlation in female X-linked Alport syndrome. Kidney Int Rep 2:850–855. https://doi.org/10.1016/j.ekir.2017.04.011
Oka M, Nozu K, Kaito H, Fu XJ, Nakanishi K, Hashimura Y, Morisada N, Yan K, Matsuo M, Yoshikawa N, Vorechovsky I, Iijima K (2014) Natural history of genetically proven autosomal recessive Alport syndrome. Pediatr Nephrol 29:1535–1544. https://doi.org/10.1007/s00467-014-2797-4
Furlano M, Martínez V, Pybus M, Arce Y, Crespí J, Venegas MDP, Bullich G, Domingo A, Ayasreh N, Benito S, Lorente L, Ruíz P, Gonzalez VL, Arlandis R, Cabello E, Torres F, Guirado L, Ars E, Torra R (2021) Clinical and genetic features of autosomal dominant Alport syndrome: a cohort study. Am J Kidney Dis 78:560-570.e1. https://doi.org/10.1053/j.ajkd.2021.02.326
Gross O, Tönshoff B, Weber LT, Pape L, Latta K, Fehrenbach H, Lange-Sperandio B, Zappel H, Hoyer P, Staude H, König S, John U, Gellermann J, Hoppe B, Galiano M, Hoecker B, Ehren R, Lerch C, Kashtan CE, Harden M, Boeckhaus J, Friede T; German Pediatric Nephrology (GPN) Study Group and EARLY PRO-TECT Alport Investigators (2020) A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport’s syndrome. Kidney Int 97:1275–1286. https://doi.org/10.1016/j.kint.2019.12.015
Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P; Study Group Members of the Gesellschaft für Pädiatrische Nephrologie, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M (2012) Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81:494–501. https://doi.org/10.1038/ki.2011.407
Yamamura T, Horinouchi T, Nagano C, Omori T, Sakakibara N, Aoto Y, Ishiko S, Nakanishi K, Shima Y, Nagase H, Takeda H, Rossanti R, Ye MJ, Nozu Y, Ishimori S, Ninchoji T, Kaito H, Morisada N, Iijima K, Nozu K (2020) Genotype-phenotype correlations influence the response to angiotensin-targeting drugs in Japanese patients with male X-linked Alport syndrome. Kidney Int 98:1605–1614. https://doi.org/10.1016/j.kint.2020.06.038
Acknowledgements
The authors would like to acknowledge all the patients and their family members who participated in this research study. We would like to thank Reata Pharmaceuticals Inc. for sharing the results of cohort 2. The authors would additionally like to thank all referring physicians from the Division of Pediatric Nephrology at Baylor College of Medicine (BCM) and Texas Children’s Hospital (TCH), as well as the staff of the BCM Human Genome Sequencing Center, especially Dr. Richard Gibbs, director, and Donna Muzny, for their contributions.
Funding
This study was supported by start-up funding from the Division of Pediatric Nephrology at Baylor College of Medicine and Texas Children’s Hospital and by funding from the Baylor College of Medicine Precision Medicine/Population Health Initiative. It was completed in collaboration with Reata Pharmaceuticals Inc., by which A. R. W. is employed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Baylor College of Medicine Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alge, J.L., Bekheirnia, N., Willcockson, A.R. et al. Variants in genes coding for collagen type IV α-chains are frequent causes of persistent, isolated hematuria during childhood. Pediatr Nephrol 38, 687–695 (2023). https://doi.org/10.1007/s00467-022-05627-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05627-w