Abstract
Background
Bardet-Biedl syndrome (BBS) is a rare, autosomal recessive ciliopathy characterized by early onset retinal dystrophy, renal anomalies, postaxial polydactyly, and cognitive impairment with considerable phenotypic heterogeneity. BBS results from biallelic pathogenic variants in over 20 genes that encode key proteins required for the assembly or primary ciliary functions of the BBSome, a heterooctameric protein complex critical for homeostasis of primary cilia. While variants in BBS1 are most frequently identified in affected individuals, the renal and pulmonary phenotypes associated with BBS1 variants are reportedly less severe than those seen in affected individuals with pathogenic variants in the other BBS-associated genes.
Case-Diagnosis
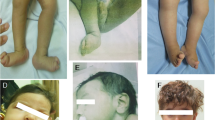
We report an infant with severe renal dysplasia and lethal pulmonary hypoplasia who was homozygous for the most common BBS1 pathogenic variant (c.1169 T > G; p.M390R) and also carried a predicted pathogenic variant in TTC21B (c.1846C > T; p.R616C), a genetic modifier of disease severity of ciliopathies associated with renal dysplasia and pulmonary hypoplasia.
Conclusions
This report expands the phenotypic spectrum of BBS with the first infant with lethal neonatal respiratory failure associated with biallelic, pathogenic variants in BBS1 and a monoallelic, predicted pathogenic variant in TTC21B. BBS should be considered among the ciliopathies in the differential diagnosis of neonates with renal dysplasia and severe respiratory failure.

Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets or analyses were generated during the current study.
Code availability
Not applicable.
References
Florea L, Caba L, Gorduza EV (2021) Bardet-Biedl syndrome-multiple kaleidoscope images: insight into mechanisms of genotype-phenotype correlations. Genes (Basel) 12:1–13
Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, Muzny DM, Young AC, Wheeler DA, Cruz P, Morgan M, Lewis LR, Cherukuri P, Maskeri B, Hansen NF, Mullikin JC, Blakesley RW, Bouffard GG, Program NCS, Gyapay G, Rieger S, Tonshoff B, Kern I, Soliman NA, Neuhaus TJ, Swoboda KJ, Kayserili H, Gallagher TE, Lewis RA, Bergmann C, Otto EA, Saunier S, Scambler PJ, Beales PL, Gleeson JG, Maher ER, Attie-Bitach T, Dollfus H, Johnson CA, Green ED, Gibbs RA, Hildebrandt F, Pierce EA, Katsanis N (2011) TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 43:189–196
Khan SA, Muhammad N, Khan MA, Kamal A, Rehman ZU, Khan S (2016) Genetics of human Bardet-Biedl syndrome, an updates. Clin Genet 90:3–15
Forsythe E, Beales PL (2013) Bardet-Biedl syndrome. Eur J Hum Genet 21:8–13
Olson AJ, Krentz AD, Finta KM, Okorie UC, Haws RM (2019) Thoraco-abdominal abnormalities in Bardet-Biedl syndrome: situs inversus and heterotaxy. J Pediatr 204:31–37
Emmanuelli V, Lahoche-Manucci A, Holder-Espinasse M, Devisme L, Vaast P, Dieux-Coeslier A, Dehennault M, Petit S, Besson R, Houfflin-Debarge V (2010) Prenatal diagnosis of hyperechogenic kidneys: a study of 17 cases. J Gynecol Obstet Biol Reprod (Paris) 39:637–646
Katsanis N, Ansley SJ, Badano JL, Eichers ER, Lewis RA, Hoskins BE, Scambler PJ, Davidson WS, Beales PL, Lupski JR (2001) Triallelic inheritance in Bardet-Biedl syndrome, a Mendelian recessive disorder. Science 293:2256–2259
Burghes AH, Vaessin HE, de La Chapelle A (2001) Genetics. The land between Mendelian and multifactorial inheritance. Science 293:2213–2214
Waters AM, Beales PL (2011) Ciliopathies: an expanding disease spectrum. Pediatr Nephrol 26:1039–1056
Capone VP, Morello W, Taroni F, Montini G (2017) Genetics of congenital anomalies of the kidney and urinary tract: the current state of play. Int J Mol Sci 18:796
Tallila J, Salonen R, Kohlschmidt N, Peltonen L, Kestila M (2009) Mutation spectrum of Meckel syndrome genes: one group of syndromes or several distinct groups? Hum Mutat 30:E813-830
Jian X, Boerwinkle E, Liu X (2014) In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic Acids Res 42:13534–13544
Putoux A, Attie-Bitach T, Martinovic J, Gubler MC (2012) Phenotypic variability of Bardet-Biedl syndrome: focusing on the kidney. Pediatr Nephrol 27:7–15
Mary L, Chennen K, Stoetzel C, Antin M, Leuvrey A, Nourisson E, Alanio-Detton E, Antal MC, Attie-Bitach T, Bouvagnet P, Bouvier R, Buenerd A, Clemenson A, Devisme L, Gasser B, Gilbert-Dussardier B, Guimiot F, Khau Van Kien P, Leroy B, Loget P, Martinovic J, Pelluard F, Perez MJ, Petit F, Pinson L, Rooryck-Thambo C, Poch O, Dollfus H, Schaefer E, Muller J (2019) Bardet-Biedl syndrome: antenatal presentation of forty-five fetuses with biallelic pathogenic variants in known Bardet-Biedl syndrome genes. Clin Genet 95:384–397
Kousi M, Soylemez O, Ozanturk A, Mourtzi N, Akle S, Jungreis I, Muller J, Cassa CA, Brand H, Mokry JA, Wolf MY, Sadeghpour A, McFadden K, Lewis RA, Talkowski ME, Dollfus H, Kellis M, Davis EE, Sunyaev SR, Katsanis N (2020) Evidence for secondary-variant genetic burden and non-random distribution across biological modules in a recessive ciliopathy. Nat Genet 52:1145–1150
Funding
This work was supported by grants from the National Institutes of Health (U01 HL134745 (FSC, JAW), R01 HL149853 (JAW)) and the Children’s Discovery Institute (FSC, JAW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was reviewed and approved by the Human Research Protection Office at Washington University School of Medicine. We obtained informed written consent from the parents for participation in this study and publication of the findings.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Viehl, L., Wegner, D.J., Hmiel, S.P. et al. Lethal neonatal respiratory failure due to biallelic variants in BBS1 and monoallelic variant in TTC21B. Pediatr Nephrol 38, 605–609 (2023). https://doi.org/10.1007/s00467-022-05616-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05616-z