Abstract
Background
Liver damage is uncommon in Shiga toxin-producing Escherichia coli–associated hemolytic uremic syndrome (STEC-HUS). Herein, we present two cases with a diagnosis of STEC-HUS that progressed to liver damage, with findings presumably related to the SERPINB11 gene c.268G > T (p.Glu90Ter) variant.
Case-diagnosis/treatment
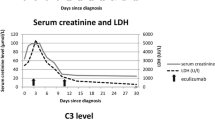
Two boys aged 3 and 2 years, respectively, were referred to our clinic with a preliminary diagnosis of STEC-HUS. The patients had low hemoglobin, thrombocyte, and haptoglobin levels but high levels of lactic dehydrogenase, urea, creatinine, and schistocytes in peripheral smears. Escherichia coli O157:H7 was detected in their stool samples. The patients underwent hemodialysis, plasma exchange, and supportive treatments. Meanwhile, cholestasis developed in the patients, resulting in elevated total bilirubin levels. During the follow-up period, kidney function recovered completely; however, liver function did not improve, and one patient developed chronic liver damage. Gene mutations that may cause liver damage were investigated, and c.268G > T (p.Glu90Ter) homozygous and heterozygous variants were detected in exon 9 of the SERPINB11 gene in the patients.
Conclusions
Our patients presented with kidney impairment and liver malfunction. Hepatic involvement in STEC-HUS may result from ischemia, hemolysis, and endothelial damage in the hepatic vessels. Liver injury in STEC-HUS cases may be associated with the homozygous SERPINB11 gene c.268G > T (p.Glu90Ter) variant.
Similar content being viewed by others
References
Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C (2017) Haemolytic uraemic syndrome. Lancet 390:681–696. https://doi.org/10.1016/S0140-6736(17)30062-4
Ylinen E, Salmenlinna S, Halkilahti J, Jahnukainen T, Korhonen L, Virkkala T, Rimhanen-Finne R, Nuutinen M, Kataja J, Arikoski P, Linkosalo L, Bai X, Matussek A, Jalanko H, Saxén H (2020) Hemolytic uremic syndrome caused by Shiga toxin-producing Escherichia coli in children: incidence, risk factors, and clinical outcome. Pediatr Nephrol 35:1749–1759. https://doi.org/10.1007/s00467-020-04560-0
de Buys Roessingh AS, de Lagausie P, Baudoin V, Loirat C, Aigrain Y (2007) Gastrointestinal complications of post-diarrheal hemolytic uremic syndrome. Eur J Pediatr Surg 17:328–334. https://doi.org/10.1055/s-2007-965013
Matthies J, Hünseler C, Ehren R, Volland R, Körber F, Hoppe B, Weber LT, Habbig S (2016) Extrarenal manifestations in Shigatoxin-associated haemolytic uremic syndrome. Klin Padiatr 228:181–188. https://doi.org/10.1055/s-0042-108444
Urushihara N, Ariki N, Oyama T, Chouda Y, Yagi T, Inoue T, Tomiyama Y, Nishiuchi R, Oda M, Tanaka N (2001) Secondary sclerosing cholangitis and portal hypertension after O157 enterocolitis: extremely rare complications of hemolytic uremic syndrome. J Pediatr Surg 36:1838–1840. https://doi.org/10.1053/jpsu.2001.28858
Vilarinho S, Choi M, Jain D, Malhotra A, Kulkarni S, Pashankar D, Phatak U, Patel M, Bale A, Mane S, Lifton RP, Mistry PK (2014) Individual exome analysis in diagnosis and management of paediatric liver failure of indeterminate aetiology. J Hepatol 61:1056–1063. https://doi.org/10.1016/j.jhep.2014.06.038
Valencia CA, Wang X, Wang J, Peters A, Simmons JR, Moran MC, Mathur A, Husami A, Qian Y, Sheridan R, Bove KE, Witte D, Huang T, Miethke AG (2016) Deep sequencing reveals novel genetic variants in children with acute liver failure and tissue evidence of impaired energy metabolism. PLoS One 11:e0156738. https://doi.org/10.1371/journal.pone.0156738
Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 37:947–954. https://doi.org/10.7326/0003-4819-137-12-200212170-00007
Seixas S, Ivanova N, Ferreira Z, Rocha J, Victor BL (2012) Loss and gain of function in SERPINB11: an example of a gene under selection on standing variation, with implications for host-pathogen interactions. PLoS One 7:e32518. https://doi.org/10.1371/journal.pone.0032518
Rakela J, Rule J, Ganger D, Lau J, Cunningham J, Dehankar M, Baheti S, Lee WM (2019) Whole exome sequencing among 26 patients with indeterminate acute liver failure: a pilot study. Clin Transl Gastroenterol 10:e00087
Riley MR, Lee KK (2004) Escherichia coli O157:H7-associated hemolytic uremic syndrome and acute hepatocellular cholestasis: a case report. J Pediatr Gastroenterol Nutr 38:352–354. https://doi.org/10.1097/00005176-200403000-00022
Gallo EG, Gianantonio CA (1995) Extrarenal involvement in diarrhoea-associated haemolytic-uraemic syndrome. Pediatr Nephrol 9:117–119. https://doi.org/10.1007/BF00858990
Silverman GA, Whisstock JC, Askew DJ, Pak SC, Luke CJ, Cataltepe S, Irving JA, Bird PI (2004) Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci 61:301–325. https://doi.org/10.1007/s00018-003-3240-3
Askew DJ, Cataltepe S, Kumar V, Edwards C, Pace SM, Howarth RN, Pak SC, Askew YS, Bromme D, Luke CJ, Whisstock JC, Silverman GA (2007) SERPINB11 is a new noninhibitory intracellular serpin. Common single nucleotide polymorphisms in the scaffold impair conformational change. J Biol Chem 282:24948–24960. https://doi.org/10.1074/jbc.M703182200
Heit C, Jackson BC, McAndrews M, Wright MW, Thompson DC, Silverman GA, Nebert DW, Vasiliou V (2013) Update of the human and mouse SERPIN gene superfamily. Hum Genomics 7:1–14. https://doi.org/10.1186/1479-7364-7-22
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was conducted in accordance with the Helsinki Declaration and ratified by the local ethics committee of Prof. Dr. Cemil Taşçıoğlu City Hospital, Istanbul, Turkey (No:17/01/2022–438).
Consent to participate
Written informed consent on behalf of the enrolled children was obtained from their parents.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Umman, N., Talip Petmezci, M., Arikan, Ç. et al. SERPINB11 variant-related liver injury in STEC-HUS: case reports and literature review. Pediatr Nephrol 37, 3243–3247 (2022). https://doi.org/10.1007/s00467-022-05602-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05602-5