Abstract
Background
Eculizumab is approved for the treatment of atypical hemolytic uremic syndrome (aHUS). Its use off-label is frequently reported. The aim of this study was to describe the broader use and outcomes of a cohort of pediatric patients exposed to eculizumab.
Methods
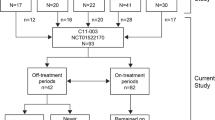
A retrospective, cohort analysis was performed on the clinical and biomarker characteristics of eculizumab-exposed patients < 25 years of age seen across 21 centers of the Pediatric Nephrology Research Consortium. Patients were included if they received at least one dose of eculizumab between 2008 and 2015. Traditional summary statistics were applied to demographic and clinical data.
Results
A total of 152 patients were identified, mean age 9.1 (+/−6.8) years. Eculizumab was used “off-label” in 44% of cases. The most common diagnoses were aHUS (47.4%), Shiga toxin-producing Escherichia coli HUS (12%), unspecified thrombotic microangiopathies (9%), and glomerulonephritis (9%). Genetic testing was available for 60% of patients; 20% had gene variants. Dosing regimens were variable. Kidney outcomes tended to vary according to diagnosis. Infectious adverse events were the most common adverse event (33.5%). No cases of meningitis were reported. Nine patients died of noninfectious causes while on therapy.
Conclusions
This multi-center retrospective cohort analysis indicates that a significant number of children and young adults are being exposed to C5 blockade for off-label indications. Dosing schedules were highly variable, limiting outcome conclusions. Attributable adverse events appeared to be low. Cohort mortality (6.6%) was not insignificant. Prospective studies in homogenous disease cohorts are needed to support the role of C5 blockade in kidney outcomes.




Similar content being viewed by others
Data Availability
Not applicable.
Code availability
Not applicable.
References
Kaplan M (2002) Eculizumab (Alexion). Curr Opin Investig Drugs 3:1017–1023
Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L (2007) Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 25:1256–1264. https://doi.org/10.1038/nbt1344
Jodele S, Dandoy CE, Lane A, Laskin BL, Teusink-Cross A, Myers KC, Wallace GH, Nelson A, Bleesing J, Chima RS, Hirsch R, Ryan TD, Benoit SW, Mizuno K, Warren M, Davies SM (2020) Complement blockade for TA-TMA: lessons learned from large pediatric cohort treated with eculizumab. Blood. https://doi.org/10.1182/blood.2019004218
Davin JC, Gracchi V, Bouts A, Groothoff J, Strain L, Goodship T (2010) Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kidney Dis 55:708–711. https://doi.org/10.1053/j.ajkd.2009.08.011
Chatelet V, Fremeaux-Bacchi V, Lobbedez T, Ficheux M, Hurault de Ligny B (2009) Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant 9:2644–2645. https://doi.org/10.1111/j.1600-6143.2009.02817.x
Gruppo RA, Rother RP (2009) Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med 360:544–546. https://doi.org/10.1056/NEJMc0809959
Nurnberger J, Philipp T, Witzke O, Opazo Saez A, Vester U, Baba HA, Kribben A, Zimmerhackl LB, Janecke AR, Nagel M, Kirschfink M (2009) Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 360:542–544. https://doi.org/10.1056/NEJMc0808527
Mache CJ, Acham-Roschitz B, Fremeaux-Bacchi V, Kirschfink M, Zipfel PF, Roedl S, Vester U, Ring E (2009) Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 4:1312–1316. https://doi.org/10.2215/CJN.01090209
Rathbone J, Kaltenthaler E, Richards A, Tappenden P, Bessey A, Cantrell A (2013) A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ Open 3:e003573. https://doi.org/10.1136/bmjopen-2013-003573
Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, Bedrosian CL, Faas SJ (2015) Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 125:3253–3262. https://doi.org/10.1182/blood-2014-09-600411
Tschumi S, Gugger M, Bucher BS, Riedl M, Simonetti GD (2011) Eculizumab in atypical hemolytic uremic syndrome: long-term clinical course and histological findings. Pediatr Nephrol 26:2085–2088. https://doi.org/10.1007/s00467-011-1989-4
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nurnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C (2013) Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368:2169–2181. https://doi.org/10.1056/NEJMoa1208981
Soliris (eculizumab) full prescribing information (2011) Alexion Pharmaceuticals, Cheshire, CT
Soliris (eculizumab) full prescribing information (2017) Boston, MA
Mahat U, Matar RB, Rotz SJ (2019) Use of complement monoclonal antibody eculizumab in Shiga toxin producing Escherichia coli associated hemolytic uremic syndrome: a review of current evidence. Pediatr Blood Cancer 66:e27913. https://doi.org/10.1002/pbc.27913
Trachtman H, Austin C, Lewinski M, Stahl RA (2012) Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol 8:658–669. https://doi.org/10.1038/nrneph.2012.196
Delmas Y, Vendrely B, Clouzeau B, Bachir H, Bui HN, Lacraz A, Helou S, Bordes C, Reffet A, Llanas B, Skopinski S, Rolland P, Gruson D, Combe C (2014) Outbreak of Escherichia coli O104:H4 haemolytic uraemic syndrome in France: outcome with eculizumab. Nephrol Dial Transplant 29:565–572. https://doi.org/10.1093/ndt/gft470
Pape L, Hartmann H, Bange FC, Suerbaum S, Bueltmann E, Ahlenstiel-Grunow T (2015) Eculizumab in typical hemolytic uremic syndrome (HUS) with neurological involvement. Medicine (Baltimore) 94:e1000. https://doi.org/10.1097/MD.0000000000001000
Percheron L, Gramada R, Tellier S, Salomon R, Harambat J, Llanas B, Fila M, Allain-Launay E, Lapeyraque AL, Leroy V, Adra AL, Berard E, Bourdat-Michel G, Chehade H, Eckart P, Merieau E, Pietrement C, Sellier-Leclerc AL, Fremeaux-Bacchi V, Dimeglio C, Garnier A (2018) Eculizumab treatment in severe pediatric STEC-HUS: a multicenter retrospective study. Pediatr Nephrol 33:1385–1394. https://doi.org/10.1007/s00467-018-3903-9
Jodele S, Fukuda T, Vinks A, Mizuno K, Laskin BL, Goebel J, Dixon BP, Teusink A, Pluthero FG, Lu L, Licht C, Davies SM (2014) Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 20:518–525. https://doi.org/10.1016/j.bbmt.2013.12.565
Fernandez C, Lario A, Fores R, Cabrera R (2015) Eculizumab treatment in a patient with hematopoietic stem cell transplantation-associated thrombotic microangiopathy and steroid-refractory acute graft versus host disease. Hematol Rep 7:6107. https://doi.org/10.4081/hr.2015.6107
Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA, Radhakrishnan J, Appel GB (2012) Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol 7:748–756. https://doi.org/10.2215/CJN.12901211
Daina E, Noris M, Remuzzi G (2012) Eculizumab in a patient with dense-deposit disease. N Engl J Med 366:1161–1163. https://doi.org/10.1056/NEJMc1112273
Vivarelli M, Pasini A, Emma F (2012) Eculizumab for the treatment of dense-deposit disease. N Engl J Med 366:1163–1165. https://doi.org/10.1056/NEJMc1111953
Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ (2013) Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol 28:1975–1981. https://doi.org/10.1007/s00467-013-2503-y
Sanchez-Moreno A, De la Cerda F, Cabrera R, Fijo J, Lopez-Trascasa M, Bedoya R, Rodriguez de Cordoba S, Ybot-Gonzalez P (2014) Eculizumab in dense-deposit disease after renal transplantation. Pediatr Nephrol 29:2055–2059. https://doi.org/10.1007/s00467-014-2839-y
Inman M, Prater G, Fatima H, Wallace E (2015) Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin Kidney J 8:445–448. https://doi.org/10.1093/ckj/sfv044
Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, Goujon JM, Fremeaux-Bacchi V, Fakhouri F (2015) Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis 65:484–489. https://doi.org/10.1053/j.ajkd.2014.09.025
Oosterveld MJ, Garrelfs MR, Hoppe B, Florquin S, Roelofs JJ, van den Heuvel LP, Amann K, Davin JC, Bouts AH, Schriemer PJ, Groothoff JW (2015) Eculizumab in pediatric dense deposit disease. Clin J Am Soc Nephrol 10:1773–1782. https://doi.org/10.2215/CJN.01360215
Lebreton C, Bacchetta J, Dijoud F, Bessenay L, Fremeaux-Bacchi V, Sellier-Leclerc AL (2017) C3 glomerulopathy and eculizumab: a report on four paediatric cases. Pediatr Nephrol 32:1023–1028. https://doi.org/10.1007/s00467-017-3619-2
Ozkaya O, Nalcacioglu H, Tekcan D, Genc G, Meydan BC, Ozdemir BH, Baysal MK, Keceligil HT (2014) Eculizumab therapy in a patient with dense-deposit disease associated with partial lipodystropy. Pediatr Nephrol 29:1283–1287. https://doi.org/10.1007/s00467-013-2748-5
Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, King KE, Kraus E, Lees LM, Melancon JK, Stewart ZA, Warren DS, Zachary AA, Montgomery RA (2009) The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant 9:231–235. https://doi.org/10.1111/j.1600-6143.2008.02451.x
Gonzalez-Roncero F, Suner M, Bernal G, Cabello V, Toro M, Pereira P, Angel Gentil M (2012) Eculizumab treatment of acute antibody-mediated rejection in renal transplantation: case reports. Transplant Proc 44:2690–2694. https://doi.org/10.1016/j.transproceed.2012.09.038
Stewart ZA, Collins TE, Schlueter AJ, Raife TI, Holanda DG, Nair R, Reed AI, Thomas CP (2012) Case report: eculizumab rescue of severe accelerated antibody-mediated rejection after ABO-incompatible kidney transplant. Transplant Proc 44:3033–3036. https://doi.org/10.1016/j.transproceed.2012.03.053
Kocak B, Arpali E, Demiralp E, Yelken B, Karatas C, Gorcin S, Gorgulu N, Uzunalan M, Turkmen A, Kalayoglu M (2013) Eculizumab for salvage treatment of refractory antibody-mediated rejection in kidney transplant patients: case reports. Transplant Proc 45:1022–1025. https://doi.org/10.1016/j.transproceed.2013.02.062
Yelken B, Arpali E, Gorcin S, Kocak B, Karatas C, Demiralp E, Turkmen A (2015) Eculizumab for treatment of refractory antibody-mediated rejection in kidney transplant patients: a single-center experience. Transplant Proc 47:1754–1759. https://doi.org/10.1016/j.transproceed.2015.06.029
Burbach M, Suberbielle C, Brocheriou I, Ridel C, Mesnard L, Dahan K, Rondeau E, Hertig A (2014) Report of the inefficacy of eculizumab in two cases of severe antibody-mediated rejection of renal grafts. Transplantation 98:1056–1059. https://doi.org/10.1097/TP.0000000000000184
Ghirardo G, Benetti E, Poli F, Vidal E, Della Vella M, Cozzi E, Murer L (2014) Plasmapheresis-resistant acute humoral rejection successfully treated with anti-C5 antibody. Pediatr Transplant 18:E1–E5. https://doi.org/10.1111/petr.12187
Orandi BJ, Zachary AA, Dagher NN, Bagnasco SM, Garonzik-Wang JM, Van Arendonk KJ, Gupta N, Lonze BE, Alachkar N, Kraus ES, Desai NM, Locke JE, Racusen LC, Segev DL, Montgomery RA (2014) Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 98:857–863. https://doi.org/10.1097/TP.0000000000000298
Rousset-Rouviere C, Cailliez M, Garaix F, Bruno D, Laurent D, Tsimaratos M (2014) Rituximab fails where eculizumab restores renal function in C3nef-related DDD. Pediatr Nephrol 29:1107–1111. https://doi.org/10.1007/s00467-013-2711-5
Chehade H, Rotman S, Matter M, Girardin E, Aubert V, Pascual M (2015) Eculizumab to treat antibody-mediated rejection in a 7-year-old kidney transplant recipient. Pediatrics 135:e551–e555. https://doi.org/10.1542/peds.2014-2275
Smith B, Kumar V, Mompoint-Williams D, Reed RD, MacLennan PA, Stegner K, Locke JE (2016) Dosing eculizumab for antibody-mediated rejection in kidney transplantation: a case report. Transplant Proc 48:3099–3105. https://doi.org/10.1016/j.transproceed.2016.03.028
Tran CL, Sethi S, Murray D, Cramer CH, Sas DJ, Willrich M, Smith RJ, Fervenza FC (2016) Discontinuation of dialysis with eculizumab therapy in a pediatric patient with dense deposit disease. Pediatr Nephrol 31:683–687. https://doi.org/10.1007/s00467-015-3306-0
Stegall MD, Chedid MF, Cornell LD (2012) The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol 8:670–678. https://doi.org/10.1038/nrneph.2012.212
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843. https://doi.org/10.2215/CJN.01640309
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Saydah SH, Xie H, Imperatore G, Burrows NR, Pavkov ME (2018) Trends in albuminuria and GFR among adolescents in the United States, 1988-2014. Am J Kidney Dis 72:644–652. https://doi.org/10.1053/j.ajkd.2018.04.021
Castaneda-Sanabria J, Hajage D, Le Jouan M, Perozziello A, Tubach F (2016) Off-label use of the expensive orphan drug eculizumab in France 2009-2013 and the impact of literature: focus on the transplantation field. Eur J Clin Pharmacol 72:737–746. https://doi.org/10.1007/s00228-016-2027-z
Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C (2016) Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89:701–711. https://doi.org/10.1016/j.kint.2015.11.026
Menne J, Delmas Y, Fakhouri F, Licht C, Lommele A, Minetti EE, Provot F, Rondeau E, Sheerin NS, Wang J, Weekers LE, Greenbaum LA (2019) Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol 20:125. https://doi.org/10.1186/s12882-019-1314-1
Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, Delmas Y, Douglas K, Furman RR, Gaber OA, Goodship T, Herthelius M, Hourmant M, Legendre CM, Remuzzi G, Sheerin N, Trivelli A, Loirat C (2015) Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 87:1061–1073. https://doi.org/10.1038/ki.2014.423
Ito S, Hidaka Y, Inoue N, Kaname S, Kato H, Matsumoto M, Miyakawa Y, Mizuno M, Okada H, Shimono A, Matsuda T, Maruyama S, Fujimura Y, Nangaku M, Kagami S (2019) Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol 23:112–121. https://doi.org/10.1007/s10157-018-1610-2
Fakhouri F, Fila M, Provot F, Delmas Y, Barbet C, Chatelet V, Rafat C, Cailliez M, Hogan J, Servais A, Karras A, Makdassi R, Louillet F, Coindre JP, Rondeau E, Loirat C, Fremeaux-Bacchi V (2017) Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 12:50–59. https://doi.org/10.2215/CJN.06440616
Jenssen GR, Vold L, Hovland E, Bangstad HJ, Nygard K, Bjerre A (2016) Clinical features, therapeutic interventions and long-term aspects of hemolytic-uremic syndrome in Norwegian children: a nationwide retrospective study from 1999 to 2008. BMC Infect Dis 16:285. https://doi.org/10.1186/s12879-016-1627-7
Vaterodt L, Holle J, Huseman D, Muller D, Thumfart J (2018) Short- and long-term renal outcome of hemolytic-uremic syndrome in childhood. Front Pediatr 6:220. https://doi.org/10.3389/fped.2018.00220
Mody RK, Gu W, Griffin PM, Jones TF, Rounds J, Shiferaw B, Tobin-D’Angelo M, Smith G, Spina N, Hurd S, Lathrop S, Palmer A, Boothe E, Luna-Gierke RE, Hoekstra RM (2015) Postdiarrheal hemolytic uremic syndrome in United States children: clinical spectrum and predictors of in-hospital death. J Pediatr 166:1022–1029. https://doi.org/10.1016/j.jpeds.2014.12.064
Rosales A, Hofer J, Zimmerhackl LB, Jungraithmayr TC, Riedl M, Giner T, Strasak A, Orth-Holler D, Wurzner R, Karch H, German-Austrian HUS Study Group (2012) Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis 54:1413–1421. https://doi.org/10.1093/cid/cis196
Rondeau E, Cataland SR, Al-Dakkak I, Miller B, Webb NJA, Landau D (2019) eculizumab safety: five-year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep 4:1568–1576. https://doi.org/10.1016/j.ekir.2019.07.016
Acknowledgements
We would like to thank the following additional centers for contributing patients to this study: Seattle Children’s Hospital, Children’s Healthcare of Atlanta, Children’s Hospital of Richmond at Virginia Commonwealth University, UT Southwestern Children’s Hospital, and Buffalo Children’s Hospital.
Funding
Dr. Sanderson is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490 and Grant 2015213 from the Doris Duke Charitable Foundation. Dr. Nester is supported by the National Institutes of Health, through Grant 1R01DK110023-01A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
IRB approval was obtained at all participating centers.
Consent to participate
This was a retrospective study, and no participant consent was required.
Consent for publication
Not applicable.
Competing interests
Carla Nester is a member of the Alexion advisory board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muff-Luett, M., Sanderson, K.R., Engen, R.M. et al. Eculizumab exposure in children and young adults: indications, practice patterns, and outcomes—a Pediatric Nephrology Research Consortium study. Pediatr Nephrol 36, 2349–2360 (2021). https://doi.org/10.1007/s00467-021-04965-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-04965-5