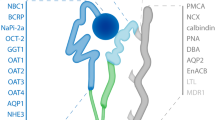
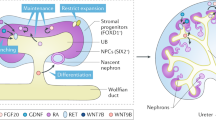
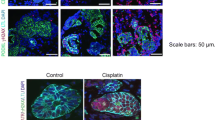
Abstract
A significant proportion of kidney disease presenting in childhood is likely genetic in origin with a growing number of genes implicated in its development. However, many children may have changes in previously undescribed or unrecognised genes. The recent development of methods for generating human kidney organoids from human pluripotent stem cells has the potential to substantially change the rate of diagnosis and the development of new treatments for some forms of genetic kidney disease. In this review, we discuss how accurately a kidney organoid models the human kidney, identifying the strengths and weaknesses of these potentially patient-derived models of renal disease.


Similar content being viewed by others
References
Fletcher J, McDonald S, Alexander SI, Australian and New Zealand Pediatric Nephrology Association (ANZPNA) (2013) Prevalence of genetic renal disease in children. Pediatr Nephrol 28:251–256. https://doi.org/10.1007/s00467-012-2306-6
Sampson MG, Robertson CC, Martini S, Mariani LH, Lemley KV, Gillies CE, Otto EA, Kopp JB, Randolph A, Vega-Warner V, Eichinger F, Nair V, Gipson DS, Cattran DC, Johnstone DB, O'Toole JF, Bagnasco SM, Song PX, Barisoni L, Troost JP, Kretzler M, Sedor JR, Nephrotic Syndrome Study Network (2016) Integrative genomics identifies novel associations with APOL1 risk genotypes in Black NEPTUNE subjects. J Am Soc Nephrol 27:814–823. https://doi.org/10.1681/ASN.2014111131
Bonomo JA, Ng MCY, Palmer ND, Keaton JM, Larsen CP, Hicks PJ, T2D-GENES Consortium, Langefeld CD, Freedman BI, Bowden DW (2014) Coding variants in nephrin (NPHS1) and susceptibility to nephropathy in African Americans. Clin J Am Soc Nephrol 9:1434–1440. https://doi.org/10.2215/CJN.00290114
Ma J, Guan M, Bowden DW, Ng MC, Hicks PJ, Lea JP, Ma L, Gao C, Palmer ND, Freedman BI (2016) Association analysis of the cubilin (CUBN) and megalin (LRP2) genes with ESRD in African Americans. Clin J Am Soc Nephrol 11:1034–1043. https://doi.org/10.2215/CJN.12971215
Nazareth D, Walshaw M (2013) A review of renal disease in cystic fibrosis. J Cyst Fibros 12:309–317. https://doi.org/10.1016/j.jcf.2013.03.005
Munro C, Ranganathan S, Coulthard K, Quinlan C (2015) Use of neutrophil gelatinase-associated lipocalin (NGAL) in CF. J Cyst Fibros 14:154. https://doi.org/10.1016/j.jcf.2014.06.010
Sanna-Cherchi S, Khan K, Westland R, Krithivasan P, Fievet L, Rasouly HM, Ionita-Laza I, Capone VP, Fasel DA, Kiryluk K, Kamalakaran S, Bodria M, Otto EA, Sampson MG, Gillies CE, Vega-Warner V, Vukojevic K, Pediaditakis I, Makar GS, Mitrotti A, Verbitsky M, Martino J, Liu Q, Na YJ, Goj V, Ardissino G, Gigante M, Gesualdo L, Janezcko M, Zaniew M, Mendelsohn CL, Shril S, Hildebrandt F, van Wijk JAE, Arapovic A, Saraga M, Allegri L, Izzi C, Scolari F, Tasic V, Ghiggeri GM, Latos-Bielenska A, Materna-Kiryluk A, Mane S, Goldstein DB, Lifton RP, Katsanis N, Davis EE, Gharavi AG (2017) Exome-wide association study identifies GREB1L mutations in congenital kidney malformations. Am J Hum Genet 101:1034. https://doi.org/10.1016/j.ajhg.2017.11.003
Westland R, Verbitsky M, Vukojevic K, Perry BJ, Fasel DA, Zwijnenburg PJ, Bökenkamp A, Gille JJ, Saraga-Babic M, Ghiggeri GM, D'Agati VD, Schreuder MF, Gharavi AG, van Wijk JA, Sanna-Cherchi S (2015) Copy number variation analysis identifies novel CAKUT candidate genes in children with a solitary functioning kidney. Kidney Int 88:1402–1410. https://doi.org/10.1038/ki.2015.239
Kohl S, Chen J, Vivante A, Hwang DY, Shril S, Dworschak GC, Van Der Ven A, Sanna-Cherchi S, Bauer SB, Lee RS, Soliman NA, Kehinde EO, Reutter HM, Tasic V, Hildebrandt F (2016) Targeted sequencing of 96 renal developmental microRNAs in 1213 individuals from 980 families with congenital anomalies of the kidney and urinary tract. Nephrol Dial Transplant 31:1280–1283. https://doi.org/10.1093/ndt/gfv447
Verbitsky M, Sanna-Cherchi S, Fasel DA, Levy B, Kiryluk K, Wuttke M, Abraham AG, Kaskel F, Köttgen A, Warady BA, Furth SL, Wong CS, Gharavi AG (2015) Genomic imbalances in pediatric patients with chronic kidney disease. J Clin Invest 125:2171–2178. https://doi.org/10.1172/JCI80877
Mallett A, Fowles LF, McGaughran J, Healy H, Patel C (2016) A multidisciplinary renal genetics clinic improves patient diagnosis. Med J Aust 204:58–59. https://doi.org/10.5694/mja15.01157
Mallett A, Corney C, McCarthy H, Alexander SI, Healy H (2015) Genomics in the renal clinic - translating nephrogenetics for clinical practice. Hum Genomics 9:1910. https://doi.org/10.1186/s40246-015-0035-1
Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M (2013) Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83:749–756. https://doi.org/10.1038/ki.2012.428
De Rechter S, Breysem L, Mekahli D (2017) Is autosomal dominant polycystic kidney disease becoming a pediatric disorder? Front Pediatr 5:272. https://doi.org/10.3389/fped.2017.00272
Ebner K, Feldkoetter M, Ariceta G, Bergmann C, Buettner R, Doyon A, Duzova A, Goebel H, Haffner D, Hero B, Hoppe B, Illig T, Jankauskiene A, Klopp N, König J, Litwin M, Mekahli D, Ranchin B, Sander A, Testa S, Weber LT, Wicher D, Yuzbasioglu A, Zerres K, Dötsch J, Schaefer F, Liebau MC, ESCAPE Study Group; GPN Study Group (2015) Rationale, design and objectives of ARegPKD, a European ARPKD registry study. BMC Nephrol 16:22. https://doi.org/10.1186/s12882-015-0002-z
Bierzynska A, McCarthy HJ, Soderquest K, Sen ES, Colby E, Ding WY, Nabhan MM, Kerecuk L, Hegde S, Hughes D, Marks S, Feather S, Jones C, Webb NJ, Ognjanovic M, Christian M, Gilbert RD, Sinha MD, Lord GM, Simpson M, Koziell AB, Welsh GI, Saleem MA (2017) Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int 91:937–947. https://doi.org/10.1016/j.kint.2016.10.013
Lieske JC, Monico CG, Holmes WS, Bergstralh EJ, Slezak JM, Rohlinger AL, Olson JB, Milliner DS (2005) International registry for primary hyperoxaluria. Am J Nephrol 25:290–296. https://doi.org/10.1159/000086360
Jayasinghe K, Quinlan C, Stark Z, Patel C, Mallawaarachchi A, Wardrop L, Kerr PG, Trnka P, Mallett AJ, KidGen Collaborative (2018) Renal genetics in Australia: kidney medicine in the genomic age. Nephrology (Carlton) 14:131S. https://doi.org/10.1111/nep.13494
Stark Z, Schofield D, Martyn M, Rynehart L, Shrestha R, Alam K, Lunke S, Tan TY, Gaff CL, White SM (2018) Does genomic sequencing early in the diagnostic trajectory make a difference? A follow-up study of clinical outcomes and cost-effectiveness. Genet Med 18:1090. https://doi.org/10.1038/s41436-018-0006-8
Stark Z, Lunke S, Brett GR, Tan NB, Stapleton R, Kumble S, Yeung A, Phelan DG, Chong B, Fanjul-Fernandez M, Marum JE, Hunter M, Jarmolowicz A, Prawer Y, Riseley JR, Regan M, Elliott J, Martyn M, Best S, Tan TY, Gaff CL, White SM, Melbourne Genomics Health Alliance (2018) Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med 20:1554–1563. https://doi.org/10.1038/gim.2018.37
Takasato M, Little MH (2015) The origin of the mammalian kidney: implications for recreating the kidney in vitro. Development 142:1937–1947. https://doi.org/10.1242/dev.104802
Lindström NO, McMahon JA, Guo J, Tran T, Guo Q, Rutledge E, Parvez RK, Saribekyan G, Schuler RE, Liao C, Kim AD, Abdelhalim A, Ruffins SW, Thornton ME, Baskin L, Grubbs B, Kesselman C, McMahon AP (2018) Conserved and divergent features of human and mouse kidney organogenesis. J Am Soc Nephrol 29:785–805. https://doi.org/10.1681/ASN.2017080887
Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, Black MJ (2018) Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27:275–283. https://doi.org/10.1016/j.ebiom.2017.12.016
Hardelin J-P, Julliard AK, Moniot B, Soussi-Yanicostas N, Verney C, Schwanzel-Fukuda M, Ayer-Le Lievre C, Petit C (1999) Anosmin-1 is a regionally restricted component of basement membranes and interstitial matrices during organogenesis: implications for the developmental anomalies of X chromosome-linked Kallmann syndrome. Dev Dyn 215:26–44
Weber S, Taylor JC, Winyard P, Baker KF, Sullivan-Brown J, Schild R, Knüppel T, Zurowska AM, Caldas-Alfonso A, Litwin M, Emre S, Ghiggeri GM, Bakkaloglu A, Mehls O, Antignac C, Network E, Schaefer F, Burdine RD (2008) SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol 19:891–903. https://doi.org/10.1681/ASN.2006111282
Combes AN, Wilson S, Phipson B, Binnie BB, Ju A, Lawlor KT, Cebrian C, Walton SL, Smyth IM, Moritz KM, Kopan R, Oshlack A, Little MH (2018) Haploinsufficiency for the Six2 gene increases nephron progenitor proliferation promoting branching and nephron number. Kidney Int 93:589–598. https://doi.org/10.1016/j.kint.2017.09.015
Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, van't Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D (2009) HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20:1123–1131. https://doi.org/10.1681/ASN.2008060633
Massa F, Garbay S, Bouvier R, Sugitani Y, Noda T, Gubler MC, Heidet L, Pontoglio M, Fischer E (2013) Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140:886–896. https://doi.org/10.1242/dev.086546
Desgrange A, Heliot C, Skovorodkin I, Akram SU, Heikkilä J, Ronkainen VP, Miinalainen I, Vainio SJ, Cereghini S (2017) HNF1B controls epithelial organization and cell polarity during ureteric bud branching and collecting duct morphogenesis. Development 144:4704–4719. https://doi.org/10.1242/dev.154336
Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, Hosawi S, Wilson S, Lawlor KT, Khan S, Oshlack A, Quinlan C, Lennon R, Little MH (2018) 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun 9:5167. https://doi.org/10.1038/s41467-018-07594-z
Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14:53–67. https://doi.org/10.1016/j.stem.2013.11.010
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16:118–126. https://doi.org/10.1038/ncb2894
Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526:564–568. https://doi.org/10.1038/nature15695
Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33:1193–1200. https://doi.org/10.1038/nbt.3392
Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6:1–13. https://doi.org/10.1038/ncomms9715
Takasato M, Er PX, Chiu HS, Little MH (2016) Generation of kidney organoids from human pluripotent stem cells. Nat Protoc 11:1681–1692. https://doi.org/10.1038/nprot.2016.098
Taguchi A, Nishinakamura R (2017) Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21:730–746.e6. https://doi.org/10.1016/j.stem.2017.10.011
Little MH, McMahon AP (2012) Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4:a008300–a008300. https://doi.org/10.1101/cshperspect.a008300
Combes AN, Zappia L, Er PX, Oshlack A, Little MH (2019) Single cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med 11:3. https://doi.org/10.1186/s13073-019-0615-0
Phipson B, Er PX, Combes AN, Forbes TA, Howden SE, Zappia L, Yen HJ, Lawlor KT, Hale LJ, Sun J, Wolvetang E, Takasato M, Oshlack A, Little MH (2019) Evaluation of variability in human kidney organoids. Nat Methods 16:79–87. https://doi.org/10.1038/s41592-018-0253-2
Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD (2018) Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23:869–881.e8. https://doi.org/10.1016/j.stem.2018.10.010
Howden SE, Thomson JA, Little MH (2018) Simultaneous reprogramming and gene editing of human fibroblasts. Nat Protoc 13:875–898. https://doi.org/10.1038/nprot.2018.007
Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, Bennetts B, Crawford J, Trnka P, Oshlack A, Patel C, Mallett A, Simons C, Little MH (2018) Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet 102:816–831. https://doi.org/10.1016/j.ajhg.2018.03.014
Tanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, Era T, Nakazato H, Nakanishi K, Sakuma T, Yamamoto T, Kurihara H, Taguchi A, Nishinakamura R (2018) Organoids from nephrotic disease-derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Rep 11:727–740. https://doi.org/10.1016/j.stemcr.2018.08.003
Boreström C, Jonebring A, Guo J, Palmgren H, Cederblad L, Forslöw A, Svensson A, Söderberg M, Reznichenko A, Nyström J, Patrakka J, Hicks R, Maresca M, Valastro B, Collén A (2018) A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell-derived kidney model for drug discovery. Kidney Int 94:1099–1110. https://doi.org/10.1016/j.kint.2018.05.003
Menon R, Otto EA, Kokoruda A, Zhou J, Zhang Z, Yoon E, Chen YC, Troyanskaya O, Spence JR, Kretzler M, Cebrián C (2018) Single-cell analysis of progenitor cell dynamics and lineage specification in the human fetal kidney. Development 145:dev164038. https://doi.org/10.1242/dev.164038
Lindström NO, De Sena Brandine G, Tran T, Ransick A, Suh G, Guo J, Kim AD, Parvez RK, Ruffins SW, Rutledge EA, Thornton ME, Grubbs B, McMahon JA, Smith AD, McMahon AP (2018) Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev Cell 45:651–660.e4. https://doi.org/10.1016/j.devcel.2018.05.010
Eriksson D, Karlsson L, Eklund O, Dieperink H, Honkanen E, Melin J, Selvig K, Lundberg J (2017) Real-world costs of autosomal dominant polycystic kidney disease in the Nordics. BMC Health Serv Res 17:560. https://doi.org/10.1186/s12913-017-2513-8
Lanktree MB, Haghighi A, Guiard E, Iliuta IA, Song X, Harris PC, Paterson AD, Pei Y (2018) Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol 29:2593–2600. https://doi.org/10.1681/ASN.2018050493
Aldridge M, Patel C, Mallett A, Trnka P (2018) Antenatally diagnosed ADPKD. Kidney Int Rep 3:1214–1217. https://doi.org/10.1016/j.ekir.2018.05.002
Freedman BS, Lam AQ, Sundsbak JL, Iatrino R, Su X, Koon SJ, Wu M, Daheron L, Harris PC, Zhou J, Bonventre JV (2013) Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol 24:1571–1586. https://doi.org/10.1681/ASN.2012111089
Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, Winston K, Tran LM, Diaz MA, Fu H, Finn LS, Pei Y, Himmelfarb J, Freedman BS (2017) Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater 16:1112–1119. https://doi.org/10.1038/nmat4994
Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS (2018) High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22:929–940.e4. https://doi.org/10.1016/j.stem.2018.04.022
Tan AY, Zhang T, Michaeel A, Blumenfeld J, Liu G, Zhang W, Zhang Z, Zhu Y, Rennert L, Martin C, Xiang J, Salvatore SP, Robinson BD, Kapur S, Donahue S, Bobb WO, Rennert H (2018) Somatic mutations in renal cyst epithelium in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29:2139–2156. https://doi.org/10.1681/ASN.2017080878
Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman M, Graf J, Bryant JC, Kleta R, Garcia A, Edwards H, Piwnica-Worms K, Adams D, Bernardini I, Fischer RE, Krasnewich D, Oden N, Ling A, Quezado Z, Zak C, Daryanani KT, Turkbey B, Choyke P, Guay-Woodford LM, Gahl WA (2010) Correlation of kidney function, volume and imaging findings, and PKHD1 mutations in 73 patients with autosomal recessive polycystic kidney disease. Clin J Am Soc Nephrol 5:972–984. https://doi.org/10.2215/CJN.07141009
Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih JH, Wolvetang EJ, McMahon AP, Holm TM, Davidson AJ (2018) A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep 11:470–484. https://doi.org/10.1016/j.stemcr.2018.06.018
Braun DA, Hildebrandt F (2017) Ciliopathies. Cold Spring Harb Perspect Biol 9:a028191. https://doi.org/10.1101/cshperspect.a028191
Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD (2012) The directed differentiation of human iPS cells into kidney podocytes. PLoS One 7:e46453. https://doi.org/10.1371/journal.pone.0046453
Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE (2017) Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. https://doi.org/10.1038/s41551-017-0069
Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, Nishinakamura R (2016) Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol 27:1778–1791. https://doi.org/10.1681/ASN.2015010096
van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ (2018) Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep 10:751–765. https://doi.org/10.1016/j.stemcr.2018.01.041
Bantounas I, Ranjzad P, Tengku F, Silajdžić E, Forster D, Asselin MC, Lewis P, Lennon R, Plagge A, Wang Q, Woolf AS, Kimber SJ (2018) Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep 10:766–779. https://doi.org/10.1016/j.stemcr.2018.01.008
Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, Cruz NM, Liu Y, Churchill AJ, Wang Y, Fu H, Pippin JW, Lin LY, Shankland SJ, Vogl AW, McNagny KM, Freedman BS (2017) Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem Cells 35:2366–2378. https://doi.org/10.1002/stem.2707
Yoshimura Y, Taguchi A, Tanigawa S, Yatsuda J, Kamba T, Takahashi S, Kurihara H, Mukoyama M, Nishinakamura R (2019) Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J Am Soc Nephrol 30:304–321. https://doi.org/10.1681/ASN.2018070747
Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K (1990) Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248:1224–1227
Lemmink HH, Mochizuki T, van den Heuvel LP, Schröder CH, Barrientos A, Monnens LA, van Oost BA, Brunner HG, Reeders ST, Smeets HJ (1994) Mutations in the type IV collagen alpha 3 (COL4A3) gene in autosomal recessive Alport syndrome. Hum Mol Genet 3:1269–1273
Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schröder CH, Smeets HJ, Reeders ST (1994) Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8:77–81. https://doi.org/10.1038/ng0994-77
Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A (2004) Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13:2625–2632. https://doi.org/10.1093/hmg/ddh284
Heide M, Huttner WB, Mora-Bermúdez F (2018) Brain organoids as models to study human neocortex development and evolution. Curr Opin Cell Biol 55:8–16. https://doi.org/10.1016/j.ceb.2018.06.006
Rayner SG, Phong KT, Xue J, Lih D, Shankland SJ, Kelly EJ, Himmelfarb J, Zheng Y (2018) Reconstructing the human renal vascular-tubular unit in vitro. Adv Healthc Mater 7:e1801120. https://doi.org/10.1002/adhm.201801120
Wilkinson LJ, Neal CS, Singh RR, Sparrow DB, Kurniawan ND, Ju A, Grieve SM, Dunwoodie SL, Moritz KM, Little MH (2015) Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric β-catenin signaling. Kidney Int 87:975–983. https://doi.org/10.1038/ki.2014.394
Acknowledgements
We thank Tom Forbes, Lorna Hale, Sara Howden, Belinda Phipson, Alexander Combes, Alicia Oshlack, Andrew Mallett, Cathelijne van den Berg, Ton Rabelink and others whose work we have referred to. We also acknowledge Dr. Chirag Patel, Dr. Andrew Mallett and the Kidgen Collaborative (www.kidgen.org.au) for the kidney gene list represented in Table 1 and in use within the Victorian Clinical Genetics Service (www.vcgs.org.au). MHL is an NHMRC Senior Principal Research Fellow with the National Health and Medical Research Council (GNT1136085).
Funding
This work was supported by the NHMRC (GNT1098654) and the Royal Children’s Hospital Foundation RenGeniPS program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Little, M.H., Quinlan, C. Advances in our understanding of genetic kidney disease using kidney organoids. Pediatr Nephrol 35, 915–926 (2020). https://doi.org/10.1007/s00467-019-04259-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04259-x