Abstract
Background
Erythropoietin-stimulating agent hyporesponsiveness (ESAH) is associated with increased cardiovascular mortality in patients with end-stage renal disease (ESRD) on hemodialysis. Dynamic treatment regimes (DTR), a clinical decision support (CDS) tool that guides the prescription of specific therapies in response to variations in patient states, have been used to guide treatment for chronic illnesses that require frequent monitoring and therapy changes. Our objective is to explore the role of utilizing a DTR to reduce ESAH in pediatric hemodialysis patients.
Methods
Retrospective analysis of ESRD patients on hemodialysis who received ESAs. Dosing was adjusted using a locally developed protocol designed to target a hemoglobin between 10 and 12 g/dl. Analyzing this protocol as a DTR, we assessed adherence to the protocol over time measuring how the hyporesponse index (ESA dose/hemoglobin value) changed due to varying levels of adherence.
Results
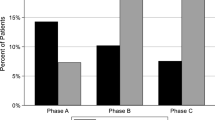
Eighteen patients met study criteria. Median hemoglobin was 11.4 g/dl (range 6.1–15.4), and median weekly ESA dose (darbepoetin-equivalent) was 0.4 mcg/kg/dose (range 0–2.1). Full adherence to the DTR was identified in 266 (71%) of the 4-week periods, with a median average adherence score of 0.80 (range 0.63–0.91). As adherence to the DTR improved, ESAH decreased. During the last 12 weeks, 13 out of 18 patients had lower average ESA/hemoglobin ratio than the first 12 weeks.
Conclusions
A DTR appears to be well-suited to the treatment of anemia in ESRD and reduces ESAH. Our work shows the potential of DTRs to drive the development and evaluation of clinical practice guidelines.



Similar content being viewed by others
References
Fadrowski JJ, Furth SL, Fivush BA (2004) Anemia in pediatric dialysis patients in end-stage renal disease network 5. Pediatr Nephrol 19:1029–1034
Jones M, Ibels L, Schenkel B, Zagari M (2004) Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int 65:757–767
Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J (2003) The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clin Ther 25:1786–1805
Zhang Y, Thamer M, Stefanik K, Kaufman J, Cotter DJ (2004) Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 44:866–876
Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK (2008) Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int 74:791–798
Borzych-Duzalka D, Bilginer Y, Ha IS, Bak M, Rees L, Cano F, Munarriz RL, Chua A, Pesle S, Emre S, Urzykowska A, Quiroz L, Ruscasso JD, White C, Pape L, Ramela V, Printza N, Vogel A, Kuzmanovska D, Simkova E, Müller-Wiefel DE, Sander A, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network (IPPN) Registry (2013) Management of anemia in children receiving chronic peritoneal dialysis. J Am Soc Nephrol 24:665–676
Lestz RM, Fivush BA, Atkinson MA (2014) Association of higher erythropoiesis stimulating agent dose and mortality in children on dialysis. Pediatr Nephrol 29:2021–2028
Fishbane S, Besarab A (2007) Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol 2:1274–1282
Chakraborty B, Murphy SA (2014) Dynamic treatment regimes. Annu Rev Stat 1:447–464
Wallace MP, Moodie EEM (2014) Personalizing medicine: a review of adaptive treatment strategies. Pharmacoepidemiol Drug Saf 23:580–585
Gillespie IA, Macdougall IC, Richards S, Jones V, Marcelli D, Froissart M, Eckardt KU, Steering Committee ARO (2015) Factors precipitating erythropoiesis-stimulating agent responsiveness in a European haemodialysis cohort: case-crossover study. Pharmacoepidemiol Drug Saf 24:414–426
Rosthøj S, Fullwood C, Henderson R, Stewart S (2006) Estimation of optimal dynamic anticoagulation regimes from observational data: a regret-based approach. Stat Med 25:4197–4215
Laber EB, Lizotte DJ, Qian M, Pelham WE, Murphy SA (2014) Dynamic treatment regimes: technical challenges and applications. Electron J Stat 8:1225–1272
Murphy SA (2003) Optimal dynamic treatment regimes. J R Stat Soc Ser B Stat Methodol 65:331–355
Cotton CA, Heagerty PJ (2011) A data augmentation method for estimating the causal effect of adherence to treatment regimens targeting control of an intermediate measure. Stat Biosci 3:28–44
United States Renal Data System (2017) 2017 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda
Author information
Authors and Affiliations
Contributions
Dr. Pollack participated in the planning, conduct, and analysis of the research, as well as writing all drafts of the manuscript. Dr. Oron developed the analytic approach and then performed a majority of the analysis and helped with writing the manuscript. Dr. Flynn was involved in reviewing the data, and with preparing and reviewing the manuscript. Dr. Munshi came up with the idea for the study, participated in reviewing the data, and contributed to the final manuscript. All authors have contributed significantly to the final manuscript.
Corresponding author
Ethics declarations
Support
This study was supported by the Seattle Children’s Center for Clinical and Translational Research Faculty Research Support Fund program. In addition, our work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002319. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pollack, A.H., Oron, A.P., Flynn, J.T. et al. Using dynamic treatment regimes to understand erythropoietin-stimulating agent hyporesponsiveness. Pediatr Nephrol 33, 1411–1417 (2018). https://doi.org/10.1007/s00467-018-3948-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-3948-9