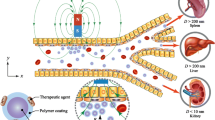
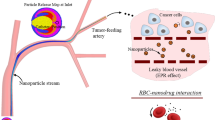
Abstract
We develop a multiscale and multiphysics computational method to investigate the transport of magnetic particles as drug carriers in blood flow under influence of hydrodynamic interaction and external magnetic field. A hybrid coupling method is proposed to handle red blood cell (RBC)-fluid interface (CFI) and magnetic particle-fluid interface (PFI), respectively. Immersed boundary method (IBM)-based velocity coupling is used to account for CFI, which is validated by tank-treading and tumbling behaviors of a single RBC in simple shear flow. While PFI is captured by IBM-based force coupling, which is verified through movement of a single magnetic particle under non-uniform external magnetic field and breakup of a magnetic chain in rotating magnetic field. These two components are seamlessly integrated within the LAMMPS framework, which is a highly parallelized molecular dynamics solver. In addition, we also implement a parallelized lattice Boltzmann simulator within LAMMPS to handle the fluid flow simulation. Based on the proposed method, we explore the margination behaviors of magnetic particles and magnetic chains within blood flow. We find that the external magnetic field can be used to guide the motion of these magnetic materials and promote their margination to the vascular wall region. Moreover, the scaling performance and speedup test further confirm the high efficiency and robustness of proposed computational method. Therefore, it provides an efficient way to simulate the transport of nanoparticle-based drug carriers within blood flow in a large scale. The simulation results can be applied in the design of efficient drug delivery vehicles that optimally accumulate within diseased tissue, thus providing better imaging sensitivity, therapeutic efficacy and lower toxicity.












Similar content being viewed by others
References
Ruenraroengsak P, Cook JM, Florence AT (2010) Nanosystem drug targeting: facing up to complex realities. J Control Release 141(3):265–276
Bae YH, Park K (2011) Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 153(3):198
Allen TM, Cullis PR (2004) Drug delivery systems: entering the mainstream. Science 303(5665):1818–1822
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2(12):751–760
Ghosh P, Han G, De M, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60(11):1307–1315
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18(9):1606–1614
Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D (1999) Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev 51(4):691–744
Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YEL, Woolliscroft MJ, Sugai JV et al (2006) Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res 12(22):6677–6686
Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9(8):615–627
McCarthy JR, Weissleder R (2008) Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev 60(11):1241–1251
Lanza GM, Yu X, Winter PM, Abendschein DR, Karukstis KK, Scott MJ, Chinen LK, Fuhrhop RW, Scherrer DE, Wickline SA (2002) Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent. Circulation 106(22):2842–2847
Li Y, Stroberg W, Lee TR, Kim HS, Man H, Ho D, Decuzzi P, Liu WK (2014) Multiscale modeling and uncertainty quantification in nanoparticle-mediated drug/gene delivery. Comput Mech 53(3):511–537
Li Y, Lian Y, Zhang LT, Aldousari SM, Hedia HS, Asiri SA, Liu WK (2016) Cell and nanoparticle transport in tumour microvasculature: the role of size, shape and surface functionality of nanoparticles. Interface Focus 6(1):20150086
Bao G, Bazilevs Y, Chung JH, Decuzzi P, Espinosa HD, Ferrari M, Gao H, Hossain SS, Hughes TJ, Kamm RD (2014) USNCTAM perspectives on mechanics in medicine. J R Soc Interface 11(97):20140301
Mitragotri S, Lahann J (2009) Physical approaches to biomaterial design. Nat Mater 8(1):15–23
Albanese A, Tang PS, Chan WC (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16
Decuzzi P, Pasqualini R, Arap W, Ferrari M (2009) Intravascular delivery of particulate systems: does geometry really matter? Pharm Res 26(1):235
Müller K, Fedosov DA, Gompper G (2014) Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci Rep 4:4871
Rohner NA, Thomas SN (2016) Flexible macromolecule versus rigid particle retention in the injected skin and accumulation in draining lymph nodes are differentially influenced by hydrodynamic size. ACS Biomater Sci Eng 3:153
Charoenphol P, Huang RB, Eniola-Adefeso O (2010) Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials 31(6):1392–1402
Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M (2010) Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release 141(3):320–327
Lee TR, Choi M, Kopacz AM, Yun SH, Liu WK, Decuzzi P (2013) On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. Sci Rep 3:2079
Xiao L, Lu G, Lu Q, Kaplan DL (2016) Direct formation of silk nanoparticles for drug delivery. Biomater ACS Sci Eng 2(11):2050
Champion JA, Katare YK, Mitragotri S (2007) Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release 121(1):3–9
Lee SY, Ferrari M, Decuzzi P (2009) Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology 20(49):495101
Champion JA, Mitragotri S (2006) Role of target geometry in phagocytosis. Proc Natl Acad Sci USA 103(13):4930–4934
Champion JA, Mitragotri S (2009) Shape induced inhibition of phagocytosis of polymer particles. Pharm Res 26(1):244–249
Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y (2013) The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid Nanofluid 14(1–2):77–87
Gentile F, Chiappini C, Fine D, Bhavane R, Peluccio M, Cheng MMC, Liu X, Ferrari M, Decuzzi P (2008) The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech 41(10):2312–2318
Decuzzi P, Lee S, Bhushan B, Ferrari M (2005) A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng 33(2):179–190
Decuzzi P, Ferrari M (2006) The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 27(30):5307–5314
Shen Z, Ye H, Kröger M, Li Y (2017) Self-assembled core–polyethylene glycol–lipid shell nanoparticles demonstrate high stability in shear flow. Phys Chem Chem Phys 19:13294
Wang S, Dormidontova EE (2010) Nanoparticle design optimization for enhanced targeting: Monte Carlo simulations. Biomacromolecules 11(7):1785–1795
Wang S, Dormidontova EE (2012) Selectivity of ligand–receptor interactions between nanoparticle and cell surfaces. Phys Rev Lett 109(23):238102
Liu J, Weller GE, Zern B, Ayyaswamy PS, Eckmann DM, Muzykantov VR, Radhakrishnan R (2010) Computational model for nanocarrier binding to endothelium validated using in vivo, in vitro, and atomic force microscopy experiments. Proc Natl Acad Sci 107(38):16530–16535
Moser BA, Steinhardt RC, Esser-Kahn A (2016) surface coating of nanoparticles reduces background inflammatory activity while increasing particle uptake and delivery. ACS Biomater Sci Eng 3:206
De M, Rana S, Akpinar H, Miranda OR, Arvizo RR, Bunz UH, Rotello VM (2009) Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nat Chem 1(6):461–465
Mout R, Moyano DF, Rana S, Rotello VM (2012) Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev 41(7):2539–2544
Anselmo AC, Mitragotri S (2017) Impact of particle elasticity on particle-based drug delivery systems. Adv Drug Deliv Rev 108:51–67
Kumar A, Graham MD (2011) Segregation by membrane rigidity in flowing binary suspensions of elastic capsules. Phys Rev E 84(6):066316
Kumar A, Graham MD (2012) Margination and segregation in confined flows of blood and other multicomponent suspensions. Soft Matter 8(41):10536–10548
Kumar A, Graham MD (2012) Mechanism of margination in confined flows of blood and other multicomponent suspensions. Phys Rev Lett 109(10):108102
Merkel TJ, Chen K, Jones SW, Pandya AA, Tian S, Napier ME, Zamboni WE, DeSimone JM (2012) The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J Control Release 162(1):37–44
Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu H, Zamboni WC, Wang AZ (2011) Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci USA 108(2):586–591
Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL, Chen M, Squires TM, Sen Gupta A, Mitragotri S (2014) Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 8(11):11243–11253
Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S (2015) Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 9(3):3169–3177
Lin Q, Huang Q, Li C, Bao C, Liu Z, Li F, Zhu L (2010) Anticancer drug release from a mesoporous silica based nanophotocage regulated by either a one- or two-photon process. J Am Chem Soc 132(31):10645–10647
Krasovitski B, Frenkel V, Shoham S, Kimmel E (2011) Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci USA 108(8):3258–3263
Kagatani S, Shinoda T, Konno Y, Fukui M, Ohmura T, Osada Y (1997) Electroresponsive pulsatile depot delivery of insulin from poly (dimethylaminopropylacrylamide) gel in rats. J Pharm Sci 86(11):1273–1277
Murakami Y, Maeda M (2005) DNA-responsive hydrogels that can shrink or swell. Biomacromolecules 6(6):2927–2929
Pankhurst QA, Connolly J, Jones S, Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36(13):R167
Peiris PM, Bauer L, Toy R, Tran E, Pansky J, Doolittle E, Schmidt E, Hayden E, Mayer A, Keri RA et al (2012) Enhanced delivery of chemotherapy to tumors using a multicomponent nanochain with radio-frequency-tunable drug release. ACS Nano 6(5):4157–4168
Schleich N, Po C, Jacobs D, Ucakar B, Gallez B, Danhier F, Préat V (2014) Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J Control Release 194:82–91
Vázquez-Quesada A, Franke T, Ellero M (2017) Theory and simulation of the dynamics, deformation, and breakup of a chain of superparamagnetic beads under a rotating magnetic field. Phys Fluids 29(3):032006
Furlani E, Ng K (2006) Analytical model of magnetic nanoparticle transport and capture in the microvasculature. Phys Rev E 73(6):061919
Tan M, Song H, Dhagat P, Jander A, Walker TW (2016) Theoretical study of alignment dynamics of magnetic oblate spheroids in rotating magnetic fields. Phys Fluids 28(6):062004
Sinha A, Ganguly R, De AK, Puri IK (2007) Single magnetic particle dynamics in a microchannel. Phys Fluids 19(11):117102
Espin M, Valverde J, Quintanilla M (2013) Stabilization of fluidized beds of particles magnetized by an external field: effects of particle size and field orientation. J Fluid Mech 732:282–303
Li W, Liu Y, Qian Z, Yang Y (2017) Evaluation of tumor treatment of magnetic nanoparticles driven by extremely low frequency magnetic field. Sci Rep 7:46287
Song W, Muthana M, Mukherjee J, Falconer RJ, Biggs CA, Zhao X (2017) Magnetic-silk core–shell nanoparticles as potential carriers for targeted delivery of curcumin into human breast cancer cells. ACS Biomater Sci Eng 3:1027
Pham AT, Zhuang Y, Detwiler P, Socolar JE, Charbonneau P, Yellen BB (2017) Phase diagram and aggregation dynamics of a monolayer of paramagnetic colloids. Phys Rev E 95(5):052607
Gontijo R, Cunha F (2017) Numerical simulations of magnetic suspensions with hydrodynamic and dipole–dipole magnetic interactions. Phys Fluids 29(6):062004
Wang S, Zhou Y, Tan J, Xu J, Yang J, Liu Y (2014) Computational modeling of magnetic nanoparticle targeting to stent surface under high gradient field. Comput Mech 53(3):403–412
Gao Y, Hulsen M, Kang T, den Toonder J (2012) Numerical and experimental study of a rotating magnetic particle chain in a viscous fluid. Phys Rev E 86(4):041503
Ravnik J, Hriberšek M (2013) High gradient magnetic particle separation in viscous flows by 3D BEM. Comput Mech 51:1–10
Tzirtzilakis E, Loukopoulos V (2005) Biofluid flow in a channel under the action of a uniform localized magnetic field. Comput Mech 36(5):360–374
Gijsen FJ, van de Vosse FN, Janssen J (1999) The influence of the non-Newtonian properties of blood on the flow in large arteries: steady flow in a carotid bifurcation model. J Biomech 32(6):601–608
Liu Y, Liu WK (2006) Rheology of red blood cell aggregation by computer simulation. J Comput Phys 220(1):139–154
Tan J, Thomas A, Liu Y (2012) Influence of red blood cells on nanoparticle targeted delivery in microcirculation. Soft Matter 8(6):1934–1946
Krüger T, Varnik F, Raabe D (2011) Efficient and accurate simulations of deformable particles immersed in a fluid using a combined immersed boundary lattice Boltzmann finite element method. Comput Math Appl 61(12):3485–3505
Krüger T, Gross M, Raabe D, Varnik F (2013) Crossover from tumbling to tank-treading-like motion in dense simulated suspensions of red blood cells. Soft Matter 9(37):9008–9015
MacMECCAN RM, Clausen J, Neitzel G, Aidun C (2009) Simulating deformable particle suspensions using a coupled lattice-Boltzmann and finite-element method. J Fluid Mech 618:13
Vahidkhah K, Diamond SL, Bagchi P (2014) Platelet dynamics in three-dimensional simulation of whole blood. Biophys J 106(11):2529–2540
Vahidkhah K, Bagchi P (2015) Microparticle shape effects on margination, near-wall dynamics and adhesion in a three-dimensional simulation of red blood cell suspension. Soft Matter 11(11):2097–2109
Ranjith SK, Patnaik B, Vedantam S (2013) No-slip boundary condition in finite-size dissipative particle dynamics. J Comput Phys 232(1):174–188
Pivkin IV, Karniadakis GE (2005) A new method to impose no-slip boundary conditions in dissipative particle dynamics. J Comput Phys 207(1):114–128
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117(1):1–19
Chen S, Doolen GD (1998) Lattice Boltzmann method for fluid flows. Annu Rev Fluid Mech 30(1):329–364
Ye H, Huang H, Sui Y, Lu XY (2016) Dynamics of a nonspherical capsule in general flow. Comput Fluids 134:31–40
Zhang J, Johnson PC, Popel AS (2008) Red blood cell aggregation and dissociation in shear flows simulated by lattice Boltzmann method. J Biomech 41(1):47–55
Sohrabi S, Liu Y (2016) A cellular model of shear-induced hemolysis. Artif Organs. https://doi.org/10.1111/aor.12832
Bernaschi M, Bisson M, Fatica M, Melchionna S, Succi S (2013) Petaflop hydrokinetic simulations of complex flows on massive GPU clusters. Comput Phys Commun 184(2):329–341
Mackay F, Ollila ST, Denniston C (2013) Hydrodynamic forces implemented into LAMMPS through a lattice-Boltzmann fluid. Comput Phys Commun 184(8):2021–2031
Fedosov DA, Caswell B, Karniadakis GE (2010) A multiscale red blood cell model with accurate mechanics, rheology, and dynamics. Biophys J 98(10):2215–2225
Dullweber A, Leimkuhler B, McLachlan R (1997) Symplectic splitting methods for rigid body molecular dynamics. J Chem Phys 107(15):5840–5851
Peskin CS (2002) The immersed boundary method. Acta Numer 11:479–517
Ye H, Huang H, Lu XY (2015) Numerical study on dynamic sorting of a compliant capsule with a thin shell. Comput Fluids 114:110
Ye H, Wei H, Huang H, Lu Xy (2017) Two tandem flexible loops in a viscous flow. Phys Fluids 29(2):021902
Zhang L, Gerstenberger A, Wang X, Liu WK (2004) Immersed finite element method. Comput Methods Appl Mech Eng 193(21):2051–2067
Liu WK, Liu Y, Farrell D, Zhang L, Wang XS, Fukui Y, Patankar N, Zhang Y, Bajaj C, Lee J et al (2006) Immersed finite element method and its applications to biological systems. Comput Methods Appl Mech Eng 195(13):1722–1749
Liu Y, Zhang L, Wang X, Liu WK (2004) Coupling of Navier–Stokes equations with protein molecular dynamics and its application to hemodynamics. Int J Numer Methods Fluids 46(12):1237–1252
Mackay F, Denniston C (2013) Coupling MD particles to a lattice-Boltzmann fluid through the use of conservative forces. J Comput Phys 237:289–298
Guo Z, Zheng C, Shi B (2002) Discrete lattice effects on the forcing term in the lattice Boltzmann method. Phys Rev E 65(4):046308
Ollila ST, Denniston C, Karttunen M, Ala-Nissila T (2011) Fluctuating lattice-Boltzmann model for complex fluids. J Chem Phys 134(6):064902
Succi S (2001) The lattice Boltzmann equation: for fluid dynamics and beyond. Oxford university press, Oxford
How T (1996) Advances in hemodynamics and hemorheology, vol 1. Elsevier, Amsterdam
Fedosov DA, Pan W, Caswell B, Gompper G, Karniadakis GE (2011) Predicting human blood viscosity in silico. Proc Natl Acad Sci USA 108(29):11772–11777
Allen MP, Tildesley DJ (1989) Computer simulation of liquids. Oxford university press, Oxford
Dao M, Li J, Suresh S (2006) Molecularly based analysis of deformation of spectrin network and human erythrocyte. Mater Sci Eng C 26(8):1232–1244
Sing CE, Schmid L, Schneider MF, Franke T, Alexander-Katz A (2010) Controlled surface-induced flows from the motion of self-assembled colloidal walkers. Proc Natl Acad Sci USA 107(2):535–540
Dünweg B, Ladd AJ (2009) Lattice Boltzmann simulations of soft matter systems. In: Holm C, Kremer K (eds) Advanced computer simulation approaches for soft matter sciences III. Springer, Berlin, pp 89–166
Suresh S, Spatz J, Mills J, Micoulet A, Dao M, Lim C, Beil M, Seufferlein T (2015) Reprint of: connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater 23:S3–S15
Fischer TM (2004) Shape memory of human red blood cells. Biophys J 86(5):3304–3313
Fischer TM (2007) Tank-tread frequency of the red cell membrane: dependence on the viscosity of the suspending medium. Biophys J 93(7):2553–2561
Skotheim J, Secomb TW (2007) Red blood cells and other nonspherical capsules in shear flow: oscillatory dynamics and the tank-treading-to-tumbling transition. Phys Rev Lett 98(7):078301
Chien S, Jan Km (1973) Ultrastructural basis of the mechanism of rouleaux formation. Microvasc Res 5(2):155–166
Zaremba L (2009) Guidance for industry and FDA staff: criteria for significant risk investigations of magnetic resonance diagnostic devices. US Department of health and human services. Food Drug Admin Center Devices Radiol Health. http://www.fda.gov/MedicalDevices/Device RegulationandGuidance/GuidanceDocuments/ucm072686.htm. Accessed 5
Franke T, Schmid L, Weitz DA, Wixforth A (2009) Magneto-mechanical mixing and manipulation of picoliter volumes in vesicles. Lab Chip 9(19):2831–2835
Petousis I, Homburg E, Derks R, Dietzel A (2007) Transient behaviour of magnetic micro-bead chains rotating in a fluid by external fields. Lab Chip 7(12):1746–1751
Acknowledgements
The authors are grateful for the support from Department of Mechanical Engineering at the University of Connecticut. Z. S. acknowledges the partial financial support from the GE Fellowship for Innovation. This research benefited in part from the computational resources and staff contributions provided by the Booth Engineering Center for Advanced Technology (BECAT) at the University of Connecticut. Part of this work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation Grant Number ACI-1053575.
Author information
Authors and Affiliations
Corresponding author
Appendix: coarse-grained potential for RBC
Appendix: coarse-grained potential for RBC
First, we use the Eq. (16) to derive the nodal force due to area constraints.
Figure 13 shows the simple triangular element of the membrane network. \(A_k\) represents the area of the triangular element. \(a_{ij} = p_i - p_j\) and i, j denotes the index from 1 to 3, \(p_i\) is the vertex points. \(\xi \) is the normal vector of the surface where the element locates, and \(\xi = \overrightarrow{a}_{21}\times \overrightarrow{a31}\), where \(\times \) is the cross product. Then we have the expression of the area of triangular element
Here, we adopt global area constraint as an example to show how to derive the nodal force. Using Eq. (16), we get
where \(\beta _a = -\,k_a(A_t-A_{t0}/A_{t0})\), subscript k represents the k-th triangular element. If we set \(\alpha = \beta _a/4A_k\), then we have the nodal force expression
Similar to the global nodal force derivation, the local nodal force can be calculated by simply setting \(\alpha = -\,k_d(A_k-A_{k0})/(4A_k-A_{k0})\).
The nodal force due to total volume constraint can be obtained
where \(V_k = \frac{\overrightarrow{\xi }^{k} \cdot \overrightarrow{b}^k}{6}\), and \(\overrightarrow{b}^k = (p_1^k+p_2^k+p_3^k)/3\) is the center of the mass of k-th triangular element. Then the nodal force could be written as
In Fig. 13b, the normal vectors of the two triangular element are \(\overrightarrow{\xi } = \overrightarrow{a}_{21}\times \overrightarrow{a}_{21}\) and \(\overrightarrow{\zeta } = \overrightarrow{a}_{34}\times \overrightarrow{a}_{24}\). \(A_1\) and \(A_2\) are the area of the two triangles, respectively. Then we can calculate the nodal force contributed by the bending potential as
As \(\theta \) is the dihedral angle, it can be expressed as \(\cos \theta = \frac{\overrightarrow{\xi }\cdot \overrightarrow{\zeta }}{|\overrightarrow{\xi }||\overrightarrow{\zeta }|}\). Then the derivation of \(\theta \) with respect to \(s_i\) is
According to this analytical expression, we can obtain the results of nodal force exerted on the four vertex points shown in Fig. 13b as following
where \(k_{11} = -\,\beta _b\frac{\cos \theta }{|\overrightarrow{\xi }|^2},k_{12} = \beta _b\frac{1}{|\overrightarrow{\xi }||\overrightarrow{\zeta }|},k_{22} = -\,\beta _b\frac{\cos \theta }{|\overrightarrow{\zeta }|^2}\), and \(\beta _b = \frac{ k_b(\sin \theta \cos \theta _0-\cos \theta \sin \theta _0)}{\sqrt{1-\cos ^2\theta }}\). Here, because \(\theta \in (0,\pi ]\), the sign of \(\sin \theta \) can be either positive or negative. It is defined by the sign of \(\mathbb {S} = (\overrightarrow{\xi }-\overrightarrow{\zeta })\cdot (\overrightarrow{b}^1-\overrightarrow{b}^2)\), where \(\overrightarrow{b}^1\) and \(\overrightarrow{b}^2\) are the vectors of center of mass of triangles 1 and 2, respectively. If \(\mathbb {S}\ge 0\), \(\sin \theta = \sqrt{1-\cos ^2\theta }\) and \(\sin \theta = -\,\sqrt{1-\cos ^2\theta }\) for \(\mathbb {S} \le 0\).
Rights and permissions
About this article
Cite this article
Ye, H., Shen, Z. & Li, Y. Computational modeling of magnetic particle margination within blood flow through LAMMPS. Comput Mech 62, 457–476 (2018). https://doi.org/10.1007/s00466-017-1508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00466-017-1508-y