Abstract
Background
Ischemia at the site of an intestinal anastomosis is one of the most important risk factors for anastomotic leakage (AL). Consequently, adequate intestinal microperfusion is essential for optimal tissue oxygenation and anastomotic healing. As visual inspection of tissue viability does not guarantee an adequate objective evaluation of intestinal microperfusion, surgeons are in dire need of supportive tools to decrease anastomotic leakage after colorectal surgery.
Methods
In this feasibility study, laparoscopic laser speckle contrast imaging (LSCI) was used to evaluate intestinal microperfusion in an experimental ischemic bowel loop model. Both large and small ischemic loops were created from the small intestine of a pig; each loop was divided into 5 regions of interest (ROI) with varying levels of ischemia. Speckle contrast and local capillary lactate (LCL) was measured in all ROIs.
Results
Both real-time visualization of intestinal microperfusion and induced perfusion deficits was achieved in all bowel loops. As a result, the emergence of regions of intestinal ischemia could be predicted directly after iatrogenic perfusion limitation, whereas without LSCI signs of decreased intestinal viability could only be seen after 30 minutes. Additionally, a significant relation was found between LCL and LSCI.
Conclusion
In conclusion, LSCI can achieve real-time intraoperative visualization of intestinal microperfusion deficits, allowing for accurate prediction of long-term postoperative ischemic complications. With this revealing capacity, LSCI could potentially facilitate surgical decision-making when constructing intestinal anastomoses in order to mitigate ischemia-related complications such as AL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Anastomotic leakage (AL) remains one of the most frequently occurring and feared complications in gastrointestinal surgery. In AL, the enteric content contaminates the peritoneal cavity and causes high morbidity and mortality rates. Despite evolving surgical techniques and improved perioperative care, the average reported incidence of AL is 10%, which has not significantly declined over the last decades ‘[1,2,3,4]. Although the exact etiology of AL remains a difficult conundrum, adequate anastomotic perfusion has been identified as one of the most important prerequisites for optimal anastomotic healing [5]. When inadequate anastomotic perfusion is left unaltered, an improvement in anastomotic perfusion from increased collateral circulation is unlikely to develop within the first five postoperative days [6], compromising anastomotic regeneration. Having an accurate indication of the local intestinal perfusion quality can help the surgeon to identify the optimal anastomotic site in order to prevent AL. As a result, intraoperative assessment of bowel perfusion is a vital element in surgical decision-making when performing restorative intestinal resections [6]. However, the traditional direct visual inspection of the intestinal tissue for signs of viability (e.g., mucosal color, pulsatile bleeding from marginal arteries) is highly subjective and does not allow for an adequate evaluation of intestinal microperfusion [6,7,8]. For this reason, numerous techniques, such as near-infrared fluorescence angiography and the assessment of various parameters of intestinal viability have recently been developed. Unfortunately, due to a lack of convincing evidence for the use of these existing techniques, there is still no general consensus regarding their clinical impact [6], leaving surgeons with a dire need for alternative tools. Horgan and Gorey defined 5 criteria for an ideal bowel viability test, namely (1) ready-to-use availability in every operating room, (2) the technique should require no other specialist personnel other than the surgeon, (3) maximum accuracy with a minimum of false-negative and, more importantly, few false positive results, (4) objectivity and reproducibility, and (5) cost-effectiveness (6).
We hypothesize that laser speckle contrast imaging (LSCI) can give an accurate indication of local intestinal perfusion quality. LSCI is a dye-free, non-contact, non-invasive, and full-field perfusion imaging technique which can deliver 2D perfusion images in real time [9]. The technique uses laser light to generate a so-called speckle pattern which changes due to motion of scatterers, or red blood cells in this case. In this study, we tested the feasibility and potential of laparoscopic LSCI for real-time intraoperative visualization of intestinal perfusion.
Materials and methods
This study was performed at the central animal facility of Maastricht University (Maastricht, The Netherlands). The animal was handled in compliance with the regulations of the Dutch legislation for animal research and the ARRIVE guidelines [10] following a protocol approved by the Experimental Animal Committee of Maastricht University (DEC-UM) under working protocol number 2017-021-001.
Animal experiment
One female Dutch landrace pig, weighing 35.5 kg, was used for the current experiment. Following an acclimatization period in the animal keeping facility, an intramuscular injection of Zolazepam/Tiletamine 6 mg/kg (Virbac, Barneveld, The Netherlands) and Thiopental 10 mg/kg (Panpharma SA, Trittau, Germany) was given as premedication. Anesthesia was induced through an intravenous injection of Sufentanyl 0.01 mg/kg/h (Hameln Pharma GmbH, Hameln, Germany), Propofol 9 mg/kg/h (B. Braun Melsungen AG, Melsungen, Germany), and Midazolam 1 mg/kg/h (Aurobindo, Baarn, The Netherlands). After intubation, the pig was continuously mechanically ventilated and anesthesia was deepened with an additional administration of Sufentanyl and Propofol when deemed necessary. At the end of the procedure, the animal was sacrificed with a lethal dose of Pentobarbital 200 mg/kg (AST Farma, Oudewater, The Netherlands).
Surgical procedure
Access to the peritoneum and the small bowel was obtained through a midline laparotomy. Four intestinal loops, with a length of approximately 15 cm, were randomly selected. At the mesenteric side of the first two loops, tissue perfusion was compromised by clipping ~ 7 peripheral arteries and veins using a vessel-clipping device (Endo Clip 10 mm Pistol Grip single use clip applier, Medtronic, Dublin, Ireland). To investigate the ability of LSCI to distinguish very subtle changes in perfusion, an additional set of two smaller loops was created. In these loops, tissue perfusion was compromised by the clipping of ~ 4 peripheral arteries and veins. In each bowel loop, 5 regions of interest (ROIs) were identified and marked with a surgical marker pen (Fig. 1). The regions were located as follows: (1 and 5) the two outer regions, located at the lateral borders of the loops, and (2 and 4) the two inner regions adjacent to 1 and 5, flanking the central region of the ischemic loop (3). This ischemic bowel loop model was a simulation of a model which was previously described [11, 12]. Images were recorded right before perfusion limitation (baseline), right after perfusion limitation (T = 0), and after 30 (T = 30) or 45 min (T = 45), respectively.
Graphic representation of the experimental setup. (a) Local capillary lactate analyzer, (b) the PerfusiX-Imaging laparoscopic laser speckle contrast imaging setup, (c) mounted laparoscope set at 15 cm above the intestine, and (d) marked positions of the regions of interest on the bowel serosa together with a dotted line indicating the vascular clipping site at the base of the central ischemic region (ROI 3). Illustration made by Sieben Medical Art, © 2022 Sieben Medical Art
Local capillary lactate levels
Local capillary lactate (LCL) levels were measured in chosen ROI by puncturing bowel serosa with a 23 Gauge needle (BD Switzerland Sarl, Eysins, Switzerland) and analyzing this blood with an EDGE lactate analyzer (ApexBio, Taipei, Taiwan, People’s Republic of China). LCL levels were obtained during all intestinal perfusion assessments.
Laparoscopic laser speckle imaging set-up and data acquisition
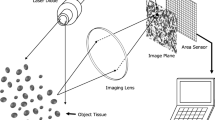
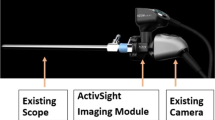
PerfusiX-Imaging (LIMIS Development BV, Leeuwarden, The Netherlands) device was used to acquire laparoscopic LSCI images [13]. PerfusiX-Imaging is a laparoscopic perfusion imager which works with standard laparoscopic equipment, namely an Olympus laparoscopic video system (OTV-S200, Olympus, Hamburg, Germany) and a 30-degree laparoscope (EndoEye, Olympus, Hamburg, Germany) in this case. It provides real-time 2D-perfusion maps instantaneously and continuously. LSCI requires a laser light source to illuminate the tissue of interest. The random interference pattern then forms an interference pattern on the camera sensor, i.e., the so-called speckle pattern. This pattern changes with movement of underlying red blood cells, in a rate which corresponds to the amount of blood flow. Hence, it is this blurring or loss in contrast which is quantified as laser speckle perfusion units (LSPU) [9]. Higher LSPU values correspond to better tissue perfusion compared to lower LSPU values. The LSPU are defined as the ratio of standard deviation divided by the mean intensity (Eq. 1).
LSCI is a fast and full-field imaging technique which can image large surfaces without the need for a contrast agent. Non-invasive subsurface perfusion measurements are characterized by means of high spatial and temporal resolution. The device houses a 639 nm laser and allows for a fast, instant switching between conventional white light and laser light. The laser was connected to the laparoscope using the unmodified optical fiber attached to the EndoEye (Fig. 1). The 2D perfusion maps were directly available in the operating room. The laparoscope was mounted onto a frame directly above the operating table at a fixed distance of 15 cm from the operating field to standardize measurement conditions and create a static optical fiber [14] (Fig. 1). The camera exposure time was 20 ms for all measurements. The aperture could not be determined. However, it was kept constant with a speckle/pixel ratio larger than one, satisfying the Nyquist criterion [15]. Images were acquired at 50 frames per second. The images were analyzed using the PerfusiX-Imaging software suite (LIMIS Development BV, Leeuwarden, The Netherlands). Images were analyzed using spatial LSCI with a spatial window size of \(7 x 7\) pixels.
On each loop, 5 ROI were marked corresponding to the vascularized (ROI 1&5), presumed marginally viable (ROI 2&4) and central ischemic (ROI 3, directly above clipped vessels) regions, respectively (Fig. 1D). LCLs were obtained by puncturing the bowel serosa in the same ROIs. The ROI size was ~ 1.5 × 1.5 cm. The average LSPU values of the ROIs of 25 frames were calculated and reported using Eq. 1.
Statistical analysis
Basic statistics were used to analyze the results. A logarithmic curve estimation was performed to test the relation between LSPU values and LCL levels.
Results
Animal experiment and surgical procedure
The surgical procedure was performed without any complications or adverse events.
Laser speckle perfusion assessment
Real-time visualization of intestinal microperfusion was achieved using PerfusiX-imaging in all bowel loops resulting in analyzable 2D speckle contrast images. The 2D perfusion maps allowed us to make a clear distinction between adequately and poorly perfused tissue regions in both large (Fig. 2a and 2d) and small ischemic loops (Fig. 2g and 2j). For larger loops, LSPU values (Fig. 2b and 2e) and corresponding perfusion maps of T = 0 seemed to predict the emergence of ischemic tissue regions, which could only be seen in white light at T = 30/T = 45. In smaller loops (Fig. 2h and 2k), visualized perfusion deficits at T = 0 were much larger than those later observed at T = 30/T = 45.
Overview of all 2D perfusion maps, corresponding white light images and the LSPU and LCL data captured from all ROIs. a Representative perfusion maps of the large ischemic bowel loop and annotated perfusion maps with ischemia indication and the location of the ROI. And corresponding white light images at baseline, 0, and 30 min of iatrogenic perfusion limitation. b Laser speckle perfusion units (LSPU) calculated for corresponding ROIs. c LCL levels in the ROIs located in a large ischemic bowel loop after 30 min of iatrogenic perfusion limitation. d Representative perfusion maps of a large ischemic bowel loop and annotated perfusion maps with ischemia indication and the location of the ROI. And corresponding white light images at baseline, 0, and 45 min of iatrogenic perfusion limitation. e LSPU calculated for corresponding ROIs. f LCL levels in the ROIs located in a large ischemic bowel loop after 45 min of iatrogenic perfusion limitation. g Representative perfusion maps of a small ischemic bowel loop and annotated perfusion maps with ischemia indication and the location of the ROI. And corresponding white light images at baseline, 0, and 30 min of iatrogenic perfusion limitation. h LSPU calculated for corresponding ROIs. i LCL levels in the ROIs located in a small ischemic bowel loop after 30 min of iatrogenic perfusion limitation. j Representative perfusion maps of a small ischemic bowel loop and annotated perfusion maps with ischemia indication and the location of the ROI. And corresponding white light images at baseline, 0, and 45 min of iatrogenic perfusion limitation. k LSPU calculated for corresponding ROIs. l LCL levels in the ROIs located in a small ischemic bowel loop after 45 min of iatrogenic perfusion limitation
Local capillary lactate levels
LCL levels in all ROIs at the time of laser speckle perfusion assessment are presented in Fig. 2c and 2f (large loops) and Fig. 2i and 2l (small loops). LCL levels below 0.60 mmol/L were expressed as ‘low’ using the lactate analyzer. For the statistical analysis, all ‘low’ LCL levels were regarded as 1 mmol/L in order to standardize and prevent underestimation. When comparing LCL levels measured in each of the ROIs within each bowel loop, no significant differences in LCL levels were found for smaller loops. However, LCL levels for larger loops did show a steep increase in LCL. The logarithmic curve estimation of the relationship between measured LSPU and LCL levels revealed a significant relation (p = 0.013) with an R-value of 0.55, indicating a moderate correlation.
Discussion
In this animal study, we successfully acquired laser speckle contrast images during intestinal surgery using the PerfusiX-Imaging laparoscopic LSCI setup. With this laparoscopic perfusion imager, we were able to detect both small and large intestinal perfusion deficits in real time. This was in contrast to visible light, which only allowed for the detection of compromised intestinal perfusion no sooner than 30 min.
Measurement of LCL levels was chosen as an objective reference for LSCI findings. LCL reflects tissue oxygenation status of intestinal cells, and its use is validated in both experimental and clinical perfusion imaging studies [11, 16, 17]. In this study we observed a clear correlation between LSPU values and lactate levels in larger ischemic loops. As anticipated, impaired perfusion was accompanied by a rise in lactate levels. However, this relation was less apparent in smaller ischemic loops, in which (relative) changes in LSPU values were not accompanied by changes in LCL. The overall logarithmic correlation of combined small and large areas could indicate that only below a critical level of perfusion, tissue becomes hypoxic, causing a steep rise in LCL. This phenomenon is commonly seen in other hemodynamic processes (e.g., hemodilution and venous-to-arterial carbon dioxide gradient related to dysoxygenation [18], i.e., an imbalance between oxygen supply and demand. Future studies into this cut-off value may support clinical decision-making to prevent irreversible tissue damage.
Observed characteristics of LSCI seem of particular interest during construction of intestinal anastomoses. In this setting, an adequate intraoperative assessment of intestinal microperfusion is required to ascertain the viability of the newly formed anastomosis to prevent ischemia-related complications such as AL. Traditional visual inspection by the surgeon has proven to be highly subjective and has little predictive value [7]. This fueled development of perfusion imaging techniques such as near-infrared fluorescence imaging, a promising technique which has gained a steep increase in popularity recent decade [19, 20]. However, a limitation of fluorescence angiography applications lies in the need for specific fluorescent dyes. Besides the fact that the injection of a dye carries certain risks (e.g., allergic reactions and toxicity), the use of dyes creates an opportunity for error in the execution and interpretation of perfusion assessment especially if repeated measurements are required. Indocyanine green (ICG) fluorescence angiography, for instance, requires the binding of ICG to blood proteins in order to visualize blood flow. When used incorrectly, ICG fluorescence angiography measures the binding of the fluorescent dye to blood proteins rather than the flow of red blood cells [21]. This could lead to a situation where the measured fluorescence intensity is caused by a difference in dye concentration rather than by a change in blood flow. Advantages of LSCI over fluorescence angiography include the ability to repetitively and continuously measurement of perfusion as there is no wash-out effect. In addition, LSCI is a full-field imaging modality and allows for real time and direct quantification of entire organ perfusion. Laparoscopic LSCI is still an uncommon perfusion imaging technique that requires further clinical validation. Next steps should include studies with regards to the ability to detect perfusion deficits in colorectal surgery and ultimately the effect of laparoscopic LSCI on anastomotic leakage rates.
Despite succeeding to detect intestinal perfusion deficits using the PerfusiX-imaging laparoscopic LSCI setup, some limitations regarding this study need to be addressed. Although we performed multiple measurements on 4 bowel loops, we only used one animal. Therefore, the data presented in this study should be treated with caution as they are limited to allow for solid conclusions. As our results contain no unreasonable outliers, we do expect to see similar results in a larger experiment. Moreover, to respect the directives on animal experimentation and the 3 R’s (Replacement, Reduction, Refinement) [22], the current set up was deemed sufficient to study the feasibility of this novel technique. The second limitation regards the choice of a small intestinal model. AL more frequently occurs in colorectal anastomoses than in small intestinal anastomoses [4], which merits a higher need for an adequate perfusion evaluation during surgery of the large intestine. Unfortunately, the spiral-like orientation of the porcine colon is not fitting for the creation of intestinal loops, hence the choice of our current model. Nevertheless, the porcine model does allow for the best translation of experimental results, as similarity to human intestinal physiology is high [23]. Another limitation is that throughout perfusion assessments in this study the distance between the camera and the bowel loops was kept constant to minimize data variations. Probably, in a clinical setting, this distance cannot be maintained at a fixed distance, which could potentially lead to altered perfusion assessment. Therefore, the system should be further studied in a minimally invasive setting, as this would be the clinical application. These studies should also focus on the normalization and quantification of the LSPU findings.
Altogether, the demonstrated feasibility of using laparoscopic LSCI to evaluate intestinal perfusion during surgery, together with the convincing results of this experimental study, trigger enthusiasm for a rapid clinical translation of this technology. However, the fine-tuning of the PerfusiX-Imaging technology, as well as additional experiments in both animal and human studies are required to further demonstrate the anticipated clinical usefulness.
Conclusion
In conclusion, laparoscopic LSCI setup can achieve a real-time intraoperative visualization of intestinal perfusion deficits, allowing for accurate prediction of long-term postoperative ischemic complications. With this revealing capacity, LSCI could potentially facilitate surgical decision-making when constructing intestinal anastomoses in order to mitigate ischemia-related complications such as AL.
References
Bosmans JWAM, Jongen ACHM, Boonen BTC, Rijn SV, Scognamiglio F, Stucchi L et al (2017) Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int J Colorectal Dis 32:305–313
Guyton KL, Hyman NH, Alverdy JC (2016) Prevention of perioperative anastomotic healing complications. Adv Surg 50(1):129–141
van Helsdingen CP, Jongen AC, de Jonge WJ, Bouvy ND, Derikx JP (2020) Consensus on the definition of colorectal anastomotic leakage: a modified Delphi study. World J Gastroenterol 26(23):3293–3303
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102(5):462–479
Colino RB, Basany EE (2018) Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta—analysis. Tech Coloproctol 22(1):15–23
Urbanavicius L, Pattyn P, de Putte DV, Venskutonis D (2011) How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg 3(5):59–69
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24(5):569–576
Al-Taher M, Pruimboom T, Schols RM, Okamoto N, Bouvy ND, Stassen LPS et al (2021) Influence of intraoperative vasopressor use on indocyanine green fluorescence angiography: first evaluation in an experimental model. Sci Rep 11(1):9650
Heeman W, Steenbergen W, van Dam G, Boerma EC (2019) Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt 24(8):1–11
Boutron I, Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol. 2020;18(7): e3000410
Al-Taher M, Barberio M, Felli E, Agnus V, Ashoka AH, Gioux S et al (2021) Simultaneous multipurpose fluorescence imaging with IRDye(R) 800BK during laparoscopic surgery. Surg Endosc 35:4840–4848
Diana M, Halvax P, Dallemagne B, Nagao Y, Diemunsch P, Charles AL et al (2014) Real-time navigation by fluorescence-based enhanced reality for precise estimation of future anastomotic site in digestive surgery. Surg Endosc 28(11):3108–3118
Heeman W, Dijkstra K, Hoff C, Koopal S, Pierie J-P, Bouma H et al (2019) Application of laser speckle contrast imaging in laparoscopic surgery. Biomed Opt Express 10(4):2010–2019
Wang J, Nadkarni SK (2014) The influence of optical fiber bundle parameters on the transmission of laser speckle patterns. Opt Express 22(8):8908–8918
Kirkpatrick SJ, Duncan DD, Wells-Gray EM (2008) Detrimental effects of speckle-pixel size matching in laser speckle contrast imaging. Opt Lett 33(24):2886–2888
Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI et al (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102(2):e169–e176
Diana M, Noll E, Diemunsch P, Moussallieh F-M, Namer I-J, Charles A-L et al (2015) Metabolism-guided bowel resection: potential role and accuracy of instant capillary lactates to identify the optimal resection site. Surgical Innovation 22(5):453–461
Lamia B, Monnet X, Teboul JL (2006) Meaning of arterio-venous PCO2 difference in circulatory shock. Minerva Anestesiol 72(6):597–604
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V et al (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259(4):700–707
D’Urso A, Agnus V, Barberio M, Seeliger B, Marchegiani F, Charles AL et al (2021) Computer-assisted quantification and visualization of bowel perfusion using fluorescence-based enhanced reality in left-sided colonic resections. Surg Endosc 35(8):4321–4331
Towle EL, Richards LM, Kazmi SM, Fox DJ, Dunn AK (2012) Comparison of indocyanine green angiography and laser speckle contrast imaging for the assessment of vasculature perfusion. Neurosurgery 71(5):1023–1030
MacArthur CJ (2018) The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br J Nutr 120(s1):S1–S7
Gonzalez LM, Moeser AJ, Blikslager AT (2015) Porcine models of digestive disease: the future of large animal translational research. Transl Res 166(1):12–27
Acknowledgements
The authors would like to thank the central animal facility of Maastricht University for their valuable assistance in conducting this animal PerfusiX-imaging experiment. The Olympus laparoscopic video system was kindly provided by Olympus. Additionally, the authors want to thank Anna Sieben for her help creating Figure 1.
Author information
Authors and Affiliations
Contributions
WH and MA designed the experimental protocol. MA, AW, and NB performed the surgical procedure and LCL sampling. WH and JC acquired imaging data. WH and JC analyzed laser speckle contrast images. WH and ECB performed statistical analysis. AW and WH wrote the main manuscript and prepared all figures and tables. All authors critically reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Drs. Joost Calon is inventor of the PerfusiX-Imaging technology and drs. Wido Heeman is part of the PerfusiX-imaging project, both authors have no financial interest in the project. Drs. Aurelia Wildeboer, dr. Mahdi Al-Taher, dr. Michele Diana, dr. Joep Derikx, dr. Christiaan Boerma, professor dr. Laurents Stassen, professor dr. Gooitzen van Dam and professor dr. Nicole Bouvy have no conflicts of interest or financial ties to enclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heeman, W., Wildeboer, A.C.L., Al-Taher, M. et al. Experimental evaluation of laparoscopic laser speckle contrast imaging to visualize perfusion deficits during intestinal surgery. Surg Endosc 37, 950–957 (2023). https://doi.org/10.1007/s00464-022-09536-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09536-9