Abstract
Background
Randomized controlled trials have been unable to demonstrate noninferiority of minimally invasive surgery for rectal cancer. The aim of this study was to assess oncologic resection success, short- and long-term morbidity, and overall survival by operative approach in a homogenous early-stage rectal cancer cohort.
Methods
This is a multicenter, propensity score-weighted cohort study utilizing deidentified data from the National Cancer Database. Individuals who underwent a formal proctectomy for early-stage rectal cancer (T1-2, N0, M0) from 2010 to 2015 were included. The primary outcome was a composite variable indicating successful oncologic resection stratified by operative approach, defined as negative margins with at least 12 lymph nodes evaluated.
Results
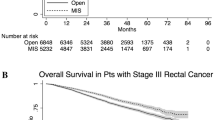
Among 3649 proctectomies for rectal adenocarcinoma, 1660 (45%) were approached open, 1461 (40%) laparoscopically, and 528 (15%) robotically. After propensity score weighting, compared to open approach, there were no differences in odds of successful oncologic resection (ORadj = 1.07, 95% CI 0.9, 1.28 and ORadj = 1.28, 95% CI 0.97, 1.7). Open approach was associated with longer mean (± SD) length of stay compared to laparoscopic (7.7 ± 0.18 vs. 6.5 ± 0.25 days, p < 0.001) and robotic (7.7 ± 0.18 vs. 6.3 ± 0.35 days, p < 0.001) approaches. In regard to 90-day mortality, compared to open approach, laparoscopic (ORadj = 0.56, 95% CI 0.36, 0.88) and robotic (ORadj = 0.45, 95% CI 0.22, 0.94) approaches were associated with a reduced odd of 90-day mortality. This mortality benefit persists in the long-term for laparoscopic approach (p = 0.003).
Conclusion
For individuals with early-stage rectal cancer treated with proctectomy, successful oncologic resection can be achieved irrespective of technical approach. Minimally invasive approaches provide short-term reduction in morbidity. Surgical approach must be tailored to each patient based on surgeon experience and judgement in collaboration with a multi-disciplinary team.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Konstantinidis IT, Ituarte P, Woo Y, Warner SG, Melstrom K, Kim J, Singh G, Lee B, Fong Y, Melstrom LG (2019) Trends and outcomes of robotic surgery for gastrointestinal (GI) cancers in the USA: maintaining perioperative and oncologic safety. Surg Endosc. https://doi.org/10.1007/s00464-019-07284-x
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Pietro BP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer the rolarr randomized clinical trial. JAMA - J Am Med Assoc 318:1569–1580. https://doi.org/10.1001/jama.2017.7219
Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam B-H, Sohn DK, Oh JH (2018) Robot-assisted versus laparoscopic surgery for rectal cancer: A Phase II open label prospective randomized controlled trial. Ann Surg 267:243–251. https://doi.org/10.1097/SLA.0000000000002321
van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218. https://doi.org/10.1016/S1470-2045(13)70016-0
Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Wilson K, Hague W, Simes J, Australasian Gastro-Intestinal Trials Group (AGITG) ALaCaRT investigators (2019) Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg 269:596–602. https://doi.org/10.1097/SLA.0000000000003021
Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H (2019) Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg 269:589–595. https://doi.org/10.1097/SLA.0000000000003002
Martínez-Pérez A, Carra MC, Brunetti F, De’Angelis N, (2017) Pathologic outcomes of laparoscopic vs open mesorectal excision for rectal cancer: A systematic review and meta-analysis. JAMA Surg 152:e165665
Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2018) Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 267:1034–1046. https://doi.org/10.1097/SLA.0000000000002523
Kolarich A, George TJ, Hughes SJ, Delitto D, Allegra CJ, Hall WA, Chang GJ, Tan SA, Shaw CM, Iqbal A (2018) Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline–directed treatment for stage II and III disease. Cancer 124:3510–3519. https://doi.org/10.1002/cncr.31527
Leo E, Belli F, Miceli R, Mariani L, Gallino G, Battaglia L, Vannelli A, Andreola S (2009) Distal clearance margin of 1 cm or less: a safe distance in lower rectum cancer surgery. Int J Colorectal Dis 24:317–322. https://doi.org/10.1007/s00384-008-0604-z
Grosek J, Velenik V, Edhemovic I, Omejc M (2017) The influence of the distal resection margin length on local recurrence and long- term survival in patients with rectal cancer after chemoradiotherapy and sphincter- preserving rectal resection. Radiol Oncol 51:169–177. https://doi.org/10.1515/raon-2016-0030
Fitzgerald TL, Brinkley J, Zervos EE (2011) Pushing the envelope beyond a centimeter in rectal cancer: oncologic implications of close, but negative margins. J Am Coll Surg 213:589–595. https://doi.org/10.1016/j.jamcollsurg.2011.07.020
Tepper JE, O’Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB, Cummings B, Gunderson L, Macdonald JS, Mayer RJ (2001) Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 19:157–163. https://doi.org/10.1200/JCO.2001.19.1.157
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol Off J Am Soc Clin Oncol 26:303–312. https://doi.org/10.1200/JCO.2007.12.7027
Friedman J (2001) Greedy function approximation: a gradient boosting machine. Ann Stat. https://doi.org/10.1214/aos/1013203451
Efron B, Hastie T, Johnstone I (2004) Least angle regression. Ann Stat. https://doi.org/10.1214/009053604000000067
McCaffrey D, Ridgeway G, Morral A (2004) Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 9(4):403–425
McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF (2013) A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 32:3388–3414. https://doi.org/10.1002/sim.5753
Ridgeway G, McCaffrey D, Morral A, Burgette L, Ann Griffin B (2017) Toolkit for Weighting and Analysis of Nonequivalent Groups: A tutorial for the twang package
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg Lond Engl 12:1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013
Jeong S-Y, Park JW, Nam BH, Kim S, Kang S-B, Lim S-B, Choi HS, Kim D-W, Chang HJ, Kim DY, Jung KH, Kim T-Y, Kang GH, Chie EK, Kim SY, Sohn DK, Kim D-H, Kim J-S, Lee HS, Kim JH, Oh JH (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15:767–774. https://doi.org/10.1016/S1470-2045(14)70205-0
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PWT, Nelson H (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III Rectal cancer on pathologic outcomes: The ACOSOG Z6051 randomized clinical trial. JAMA 314:1346–1355. https://doi.org/10.1001/jama.2015.10529
Stevenson ARL, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J, ALaCaRT Investigators, (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT randomized clinical trial. JAMA 314:1356–1363. https://doi.org/10.1001/jama.2015.12009
Vennix S, Pelzers L, Bouvy N, Beets GL, Pierie J-P, Wiggers T, Breukink S (2014) Laparoscopic versus open total mesorectal excision for rectal cancer. In: Breukink S (ed) Cochrane Database of Systematic Reviews. John Wiley & Sons Ltd, Chichester UK, p CD05200
Quintana JM, Anton-Ladislao A, Lázaro S, Gonzalez N, Bare M, de Larrea NF, Redondo M, Briones E, Escobar A, Sarasqueta C, Garcia-Gutierrez S, Group for the R-C (2017) Outcomes of open versus laparoscopic surgery in patients with rectal cancer. Int J Colorectal Dis. https://doi.org/10.1007/s00384-017-2925-2
Kang S-B, Park JW, Jeong S-Y, Nam BH, Choi HS, Kim D-W, Lim S-B, Lee T-G, Kim DY, Kim J-S, Chang HJ, Lee H-S, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim D-H, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645. https://doi.org/10.1016/S1470-2045(10)70131-5
Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gullà N, Noya G, Boselli C (2012) Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis 14:e134–e156. https://doi.org/10.1111/j.1463-1318.2011.02907.x
Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J (2015) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a Meta-analysis of eight studies. J Gastrointest Surg 19:516–526. https://doi.org/10.1007/s11605-014-2697-8
Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2017) Robotic versus laparoscopic minimally invasive surgery for rectal cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000002523
Allaix ME, Furnée E, Esposito L, Mistrangelo M, Rebecchi F, Arezzo A, Morino M (2018) Analysis of early and long-term oncologic outcomes after converted laparoscopic resection compared to primary open surgery for rectal cancer. World J Surg 42:3405–3414. https://doi.org/10.1007/s00268-018-4614-x
Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP (2017) Using the national cancer database for outcomes research a review. JAMA Oncol 3:1722–1728. https://doi.org/10.1001/jamaoncol.2016.6905
Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009) - CEBM. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed 21 Dec 2018
Funding
There were no sources of funding for this research.
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the conception of the manuscript. Dr. Kethman acquired and analyzed the data and Drs. Kethman, Dietz, Stein, and Steinhagen aided in the interpretation of the data and analysis. Dr. Kethman was responsible for drafting initial drafts of the manuscript and Drs. Bingmer, Ofshteyn, Charles, Stein, Dietz, and Steinhagen contributed to critically revising important intellectual content. All authors gave final approval of the published version of the manuscript and all agree to be accountable for all aspects of the manuscript.
Corresponding author
Ethics declarations
Disclosures
Drs. William Kethman, Katherine Bingmer, Asya Ofshteyn, Ronald Charles, and Emily Steinhagen have no conflicts of interest or financial ties, relevant to the material in this manuscript, to disclose. Dr. Sharon Stein has received a Physician’s Foundation grant for work unrelated to the topic of this manuscript and support from Stryker Corporation for travel expenses. Dr. David Dietz has received compensation as faculty for an educational course with Johnson & Johnson – Medical Device Business Services, Inc..
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kethman, W.C., Bingmer, K.E., Ofshteyn, A. et al. Effects of surgical approach on short- and long-term outcomes in early-stage rectal cancer: a multicenter, propensity score-weighted cohort study. Surg Endosc 36, 5833–5839 (2022). https://doi.org/10.1007/s00464-022-09033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09033-z