Abstract
Background
Recent evidences suggest that gallbladder drainage is the treatment of choice in elderly or high-risk surgical patients with acute cholecystitis (AC). Despite better outcomes compared to other approaches, endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) is burdened by high mortality. The aim of the study was to evaluate predictive factors for mortality in high-risk surgical patients who underwent EUS-GBD for AC.
Methods
A retrospective analysis of a prospectively maintained database was performed. Electrocautery-enhanced lumen-apposing metal stents were used; all recorded variables were evaluated as potential predictive factors for mortality.
Results
Thirty-four patients underwent EUS for suspected AC and 25 (44% male, age 78) were finally included. Technical, clinical success rate and adverse events rate were 92%, 88%, and 16%, respectively. 30-day and 1-year mortality were 12% and 32%. On univariate analysis, age-adjusted Charlson Comorbidity Index (CCI) (OR 20.8[4–68.2]), acute kidney injury (AKI) (OR 21.4[2.6–52.1]) and clinical success (OR 8.9[1.2–11.6]) were related to 30-day mortality. On multivariate analysis, CCI and AKI were independently related to long-term mortality. Kaplan–Meier curves showed an increased long-term mortality in patients with CCI > 6 (hazard ratio 7.6[1.7–34.6]) and AKI (hazard ratio 11.3[1.4–91.5]).
Conclusions
Severe comorbidities and AKI were independent predictive factors confirming of long-term mortality after EUS-GBD. Outcomes of EUS-GBD appear more influenced by patients’ conditions rather than by procedure success.
Similar content being viewed by others
Biliary stone disease represents one of the most frequent conditions with a worldwide prevalence ranging between 15 and 20% [1, 2]. Most reported complications are acute cholecystitis (AC), pancreatitis, jaundice, and cholangitis, with a cumulative incidence of 0.1–4% per year. Risk factors for biliary stone disease and its complications are female gender, age, pregnancy and puerperium, obesity, comorbidities (such as liver cirrhosis, Crohn's disease or hemolytic anemia) and concomitant medications (such as hormone therapy, parenteral nutrition or somatostatin analogs) [1, 3, 4] .
AC is the most common complication of biliary stone disease, with an incidence of 10% in symptomatic population. While there is a wide consensus regarding early laparoscopic cholecystectomy as the treatment of choice for AC in patients fit for surgery [5, 6], recommendations on the management of elderly and/or high-risk surgical patients are based on low grade evidence and as thus not completely unanimous. Nevertheless, Tokyo 2018 guidelines introduced the concept that treatment should be based on the evaluation of AC severity, patient's general status and comorbidities [7]. Despite conclusive results are lacking on the best management (surgery vs drainage), the authors of the guidelines recommend biliary drainage for severe AC and for high-risk surgical patients with moderate AC.
Three different strategies are available for gallbladder drainage (GBD) including percutaneous transhepatic-GBD, endoscopic transpapillary GBD (during endoscopic retrograde cholangiopancreatography ERCP) and endoscopic ultrasound (EUS)-guided GBD. While PT-GBD is considered the standard drainage method due to its general availability, the two endoscopic approaches require specific expertise that is usually available only in high-volume centers [8]. Endoscopic transpapillary GBD is burdened by a low rate of technical and clinical success rate, due to the difficulty of cannulation and stent placement in an obstructed cystic duct, and the risk of post-ERCP acute pancreatitis [9, 10]. In the last years, thanks to technical and technological improvements, in primis due to the development of dedicated devices, several studies reported good technical and clinical outcomes of EUS-GBD in high-risk surgical patients with AC; [11, 12] recently, results from a randomized trial definitely demonstrated that EUS-GBD with lumen-apposing metal stent (LAMS) is superior over PT-GBD in terms of 30-day adverse events and long-term outcomes (adverse events, recurrent AC, and need for reintervention). [13]
However, despite promising results, EUS-GBD is still far from being a perfect strategy in this setting; indeed, a recent meta-analysis reported a 26% all-cause mortality rate despite very high technical and clinical success rate (> 95%). [10] Therefore, the identification of predictive factors for short and long-term mortality could improve patient selection and consequently overall clinical outcomes.
The aim of our study was to evaluate the outcomes of EUS-GBD with LAMS in high-risk surgical patients with AC. The main goal was to identify predictive factors for short-term and long-term mortality; we also focused on EUS-GBD procedural management (sedation, airways intubation) and hospitalization (referral department, need for ICU admission and ICU length of stay).
Materials and methods
Study design
A retrospective analysis of a prospectively maintained database was conducted; consecutive high-risk surgical patients who underwent EUS for AC from January 2017 to December 2019 were considered. Inclusion criteria were: (a) Suspected AC complicated by biliary sepsis; (b) surgical contraindication agreed on multidisciplinary team (MDT) evaluation; (c) patients who underwent EUS with therapeutic intent (EUS-GBD); (d) written informed consent. Exclusion criteria were: (a) no EUS sign of biliary inflammation or AC; (b) common bile duct stones with cholangitis; (c) post-surgical altered upper gastrointestinal anatomy; (d) previous gallbladder drainage.
All cases underwent computed tomography (CT) before EUS and were discussed by the Hospital Multidisciplinary Team, including at least one gastroenterologist, surgeon, oncologist, and radiologist. Written informed consent for interventional EUS procedure was obtained, clearly specifying the use of LAMS for EUS-GBD. The study was conducted according to local policy on retrospective studies and to the principles of the Declaration of Helsinki (revision of Edinburgh 2000).
The following variables were recorded: patients' age and gender, comorbidities and concomitant medications, ASA class, age-adjusted Charlson Comorbidities Index (CCI) [14, 15], size of LAMS, type of sedation administered, airways intubation, severity of AC according to Tokyo guidelines [16, 17], C reactive protein, referral department, ICU admission and length of stay (LoS), hospital LoS.
Technical success was defined as successful LAMS placement with confirmed bile drainage. Clinical success was defined as improvement of clinical and biochemical parameters within 1 week after EUS-GBD. Adverse events were described and graded according to endoscopic adverse events classification [18] Recurrence of AC have been recorded. Patients were followed up for 12 months after EUS-GBD or until death.
EUS-GBD procedure
All EUS-GBD were conducted in our endoscopic suite by two experienced operators (A.L and P.F., with more than 20 EUS-GBD each). A curvilinear-array (GF-UCT-180, Olympus comp.) or a forward-view (TGF-UC180J, Olympus Comp.) echoendoscope with a dedicated ultrasound processor (EU-ME2, Olympus comp.) were used based on operator’s choice. [19] Electrocautery-enhanced LAMS delivery system (Hot-Axios, Boston Scientific Medical Corporation, Marlborough, USA) was used in conjunction with the ERBE VIO 300D electrosurgical unit using pure cut mode (AUTOCUT mode, effect 5, power 100 W). Procedures were done under EUS guidance without fluoroscopic assistance. Transduodenal drainage was preferred, while transgastric route was considered after failure of the attempt to identify an adequate transduodenal window.
Percutaneous transhepatic gallbladder drainage
Patients requiring PT-GBD were referred to the Interventional Radiology Unit, S.Orsola-Malpighi Hospital in Bologna (Italy), located at a distance of 45 km from the Hospital of Imola. The decision to refer the patient for EUS- or PT-GBD was based on local expertise and availability, the need to obtain immediate gallbladder drainage and when the patient was not considered safely transportable, based on the underlying clinical condition.
Sedation and airways management
All procedures were performed with anesthesiologists’ assistance. Patients were placed in left lateral decubitus, under continuous cardiopulmonary parameter monitor. The type of sedation administered (conscious sedation, deep sedation, or general anesthesia) was chosen by the anesthesiologist based on patients' hemodynamic and respiratory status; spontaneous breathing was usually preferred, while airways intubation was reserved to most compromised cases.
Clinical follow-up and stent removal
Oral feeding with a liquid diet was resumed after 12 h. Antibiotic treatment was maintained until clinical and biochemical resolution of AC. No LAMS removal was planned because of advanced age and/or severe comorbidities. Biliary symptoms and adverse events were recorded for 12 months or until death occurrence.
Statistical analysis
Categorical variables were reported as number and percentage, n (%) and compared using the Fischer exact test or the Chi-square test, when appropriate. Normally distributed continuous variables were reported as means ± standard deviation (means ± SD) and compared using T-test; non-normally distributed variables were reported as median and interquartile range (median [IQR]) and compared using Mann–Whitney test. An “intention-to-treat” analysis was conducted, including all patients with AC who underwent an EUS-GBD attempt. Receiver Operating Characteristic (ROC) curves were analyzed to identify the best cut-off values. Logistic regression model were used to identify variables related to 30-day and 1-year mortality; multivariate analysis was used to identify variables independently related to those outcomes; results were expressed as odds ratio (OR) and 95% confidence intervals (95% CI). Cox-proportional hazard regression was used to assess hazard ratio (HR) and 95% CI of factors related to long-term mortality. Kaplan–Meier curves were used to compare long-term survivals. P values < 0.05 were considered statistically significant. MedCalc Statistical Software version 19 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019) was used.
Results
Study population
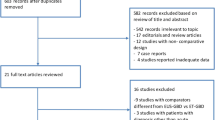
During the study period, 11 patients were referred to Interventional Radiology Unit and 8 underwent PT-GBD, while thirty-four consecutive high-risk surgical patients with suspected AC underwent EUS. Nine patients were excluded from the analysis because either no EUS signs of AC were seen (no. 6), or common bile duct stones were found (no. 3). Finally, the remaining 25 patients with AC entered the intention-to-treat analysis. Study flow-chart is shown in Supplementary Fig. 1. Baseline characteristics of the included patients with confirmed AC included in which EUS-GBD was indicated and attempted is reported in Table 1.
In detail, 11 (44%) patients were male with a median age of 78 [75–88] years. All patients presented moderate to severe AC according to Tokyo guidelines classification. Mean age-adjusted CCI was 6.1 ± 1.4. At the time of presentation, 7 patients (28%) fulfilled the criteria for an acute kidney injury (AKI) before EUS-GBD [17].
Hospitalization
Thirteen patients (52%) were referred to EUS-GBD from the internal medicine department and 8 (32%) from the surgical department; only 4 (16%) patients required admission to the ICU. No patient required admission to ICU after EUS-GBD; therefore, the overall ICU admission rate was 16%. Median ICU length of stay was 5 [3.5–9.5] days (Table 2). The overall length of stay was 9 [7,8,9,10,11] days. In-hospital mortality was 12% (Table 3).
Sedation and airways management
While in most cases (no. 14, 56%), deep sedation with intravenous propofol was used, in 8 patients (32%) EUS-GBD was completed under conscious sedation (fentanyl plus midazolam). Only 3 patients (12%) required general anesthesia with airways intubation. In two cases, tracheal intubation became necessary during the procedure, after sedation induction, while in the remaining case general anesthesia was preferred by the anesthesiologist before the procedure. All patients regained spontaneous breathing in the endoscopic suite.
EUS-GBD
Technical success was achieved in 23 out of 25 patients (92%). In two cases of technical failure, the operators were not able to identify an operative window for gallbladder drainage; in both cases, switching the linear to a forward-view echoendoscope was not successful either (Fig. 1). In both cases, PT-GBD was planned within 48-h; however, the patients showed clinical and biochemical improvement with fasting, intravenous liquid and antibiotics; no percutaneous drainage was then performed and both patients were alive at the end of follow-up. One patient, despite technically successful EUS-GBD, did not recover from biliary sepsis due to AC. Overall, clinical success was achieved in 22 out of 25 patients (88%).
The electrocautery-enhanced LAMS delivery system was used under sole EUS guidance with the direct puncture technique in all cases. In 20 cases (80%) a 10 × 10 mm LAMS was used; in 4 cases (16%) a 15 × 10 mm LAMS; in the remaining case (4%) an 8 × 8 mm was used after the misdeployment of a 10 × 10 mm one [20]. Transduodenal EUS-GBD was performed in 18 cases (78%) and transgastric in the remaining 5 cases after failure to identify an adequate transduodenal window (22%). Median procedure time was 10 min [8.5–16]; procedure time was significantly higher in transgastric drainage than transduodenal ones (20 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] vs. 9.5 [5,6,7,8,9,10,11,12,13,14,15,16], respectively; P = 0.05).
We observed three procedural adverse events in two patients (8% of patients included in the intention-to-treat analysis; 9% in the per-protocol analysis). One patient presented bleeding after stent deployment; the bleeding did not require hemostatic treatment and a coaxial 6Fr double pig-tail plastic catheter was left inside the LAMS. One patient (4%) presented with recurrent AC with cholangitis and sepsis 3 weeks after EUS-GBD; on CT and upper endoscopy, significant tissue overgrowth was observed on the LAMS. A fully covered biliary self-expandable metal stent was then placed through the obstructed LAMS, achieving good biliary drainage. Subsequently, the patient underwent elective cholecystectomy because of persistent biliary symptoms. The patient was alive at the end of follow-up. [21] In all other cases LAMS were left in place indefinitely and no other patient required surgery thereafter. One patient (4%) suffered an ischemic stroke two days after EUS-GBD after aspirin withdrawal. The patient survived AC with major neurological sequelae (hemiplegia and dysphagia). Data are reported in Table 3.
Mortality after EUS-GBD
Patients were followed up for a median of 12 [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] months. Thirty-day mortality and 1-year mortality were 12% and 32%, respectively (Table 3). 30-day mortality causes were biliary sepsis due to AC in one case and progressive heart and renal failure in two cases. 1-year mortality causes were considered related to AC in 1 case (biliary sepsis), possibly related to AC in 1 case (sepsis of unknown origin 5 months after EUS-GBD) and unrelated to AC in the remaining 6 cases (no. 4 cardiovascular, no. 1 stroke, no. 1 hip fracture).
On univariate analysis, age-adjusted CCI (OR 20.8 [4–68.2]), AKI (OR 21.4 [2.6–52.1]) and fail to achieve clinical success (OR 8.9 [1.2–11.6]) were related to 30-day mortality, while no variable was independently related to 30-day mortality on multivariate analysis (Table 4).
On univariate analysis, ASA class IV (OR 6.2 [1.0–62.1]), age-adjusted CCI (OR 9.8 [1.4–68.8]), AKI (OR 48.0 [3.6–631.8]) and concomitant antiplatelet agents (OR 7.2 [1.1–48.6]) were associated to 1-year mortality; C reactive protein (OR 0.89 [0.80–0.99]) appeared inversely related to 1-year mortality. On multivariate analysis, age-adjusted CCI (OR 3.2 [1.2–11.8]) and AKI (OR 28.5 [2.0–467.8]) were independently related to 1-year mortality.
Long-term survival analysis
Receiver Operating Characteristic (ROC) curves (Supplementary Fig. 2) identified age-adjusted CCI > 6 as the best cut-off value for mortality after EUS-GBD (area under ROC curve 0.92 [0.74–0.99]; P < 0.001). The identified cut-off (age-adjusted CCI > 6) showed a sensitivity of 100% and a specificity of 68.2%.
Cox-proportional regression analysis (Supplementary Table 1) showed that C reactive protein was inversely related to long-term mortality (Exp (b) 0.92 [0.85–0.99]); on multivariate analysis, age-adjusted CCI > 6 (Exp (b) 2.4 [1.1–5.5]) and AKI (Exp (b) 11.3 [1.4–91.5]) were independently related to long-term mortality.
Kaplan–Meier curves (Fig. 2A, B) showed the long-term patients’ survival according to an age-adjusted CCI > 6 (hazard ratio 7.6 [1.7–34.6]; P = 0.009) and according to the presence of AKI (hazard ratio 11.3 [1.4–91.5]; P < 0.001).
Discussion
It was recently demonstrated that EUS-GBD could be considered one of the treatment choices in high-risk surgical patients with AC [13, 22]. Despite better outcomes when compared to PT-GBD and ET-GBD, EUS-GBD cannot be still considered the golden bullet as it is burdened by high mortality rate [10]. We hypothesize that the identification of predictive factors for mortality could help to identify those patients who may benefit from EUS-GBD, leading to an indirect improvement in clinical outcomes. Our study identified age-adjusted CCI and presence of AKI as independent predictors of mortality in this setting. In our population, if an age-adjusted CCI > 6 cut-off was adopted, a sevenfold increase in mortality was observed (Fig. 2A); similarly, if a patient fulfilled the criteria for AKI before EUS-GBD, an 11-fold increase risk in mortality was expected (Fig. 2B).
Among other predictive factors identified in our analysis, C reactive protein appeared inversely correlated to 1-year mortality. This finding was confirmed on univariate Cox-proportional regression analysis but, in both cases, was not included in the multivariate model and identified as independent predictive factors (Table 4). In other terms, a lower C reactive protein value on admission could be interpreted as a risk factor for long-term mortality. In our opinion, this unexpected result could be interpreted as an increased mortality in patients with more compromised status; in this case, the lower C reactive protein should represent an impaired liver synthesis rather than a mild systemic inflammation. Interestingly, while EUS-GBD clinical success was related to 30-day mortality, the long-term survival did not appear to be affected by the results of biliary drainage. In other words, the factors influencing high mortality after EUS-GBD were not related to the drainage itself but to the underlying patients’ conditions.
The outcome of frail patients with AC could be improved through careful treatment allocation [23, 24]. In this field, several issues are still debated; for example, a recent trial demonstrated that lap-cholecystectomy reduced the risk of complications compared to PT-GBD; [25] however, a systematic review and meta-analysis suggested that delayed cholecystectomy after temporary PT-GBD seems to guarantee better surgical outcomes over emergency cholecystectomy [26]. While high-quality data (results from the DRAC-1 trial) suggested that EUS-GBD is the technique of choice for gallbladder drainage [13], prospective studies should demonstrate if this advantage is confirmed even in patients with severe impairment of general conditions.
Among the elderly and frail patients, only 16% required ICU admission and 12% general anesthesia with airway intubation (Table 2); moreover, all patients regained spontaneous breathing in the endoscopic suite and none presented anesthesiological complications or required ICU admission after the procedure.
These data corroborate recent advances suggesting that EUS-GBD should be considered the treatment of choice in high-risk surgical patients with AC [9, 10, 13, 22]. First of all, technical and clinical success rate are comparable to PT-GBD. Moreover, a recent RCT demonstrates that patients treated with EUS-GBD have a lower risk of adverse events and recurrence of AC. [13] Thirdly, even in frail and elderly patients, EUS-GBD could be performed without general anesthesia, with a dramatic reduction in need for ICU admission. Finally, EUS-GBD could even be considered a definitive treatment that potentially allows rapid patient discharge with no further intervention required. For all these reasons, in particular during the Covid-19 crisis, the multidisciplinary team of our hospital has expanded EUS-GBD indications in order to face with the dramatic shortage of hospital ICU beds and operating rooms [27, 28].
We acknowledge that this study has several limitations. The main limitation is related to the study design; despite the inclusion of all the consecutive high-risk surgical patients with AC who underwent an EUS-GBD attempt, the retrospective analysis could involve potential bias. We tried to limit any selection bias, including only patients who were considered for EUS-GBD. Indeed, a comparison between EUS-GBD and other treatments was not the aim of this study. Despite the advantage of a homogeneous setting in a single-center study, the relatively small sample size is a counterpart that has to be taken into account. The number of patients included, and events observed could have limited multivariate analysis of predictive factors for 30-day mortality. However, our sample size appeared adequate to conduct a multivariate analysis on predictive factors for long-term mortality. Finally, we tried to overcome the intrinsic limitation of a retrospective study with an intention-to-treat analysis. In fact, we included in the analysis all the patients in which EUS-GBD was indicated and attempted; this method could in part justify the quite different results to other series in terms of clinical success rate and 30-day mortality [22].
We preferred to conduct a single-arm study rather than a comparison to patients who underwent PT-GBD since the chose was based on local expertise, availability, the need to obtain immediate gallbladder drainage, and when the patient was not considered safely transportable. We considered that a comparison between the two groups (EUS-GBD vs PT-GBD) could be burdened by significant selection bias.
This is the first study aiming to correlate clinical predictive factors with short and long-term mortality after EUS-GBD performed for AC. We acknowledge that the results of a single study may not constitute sufficient evidence to change current management; however, we believe that this study provides new implications and suggestions. In particular, after the analysis of our population, we have reconsidered the indications for EUS-GBD in patients with age-adjusted CCI greater than 7. The new approach led to a reduction in mortality and adverse events in 2020 since patients with severe comorbidities or compromised health status were initially treated just with PT-GBD. At a later stage, the same subjects were re-evaluated by the multidisciplinary team considering either surgery or PT-GBD removal with/without conversion to EUS-GBD. This simple clinical algorithm could improve EUS-GBD outcomes on one hand and spare futile and expensive procedures on the other hand.
In conclusion, our study is consistent with other trials that confirmed the role of EUS-GBD as the treatment of choice in high-risk surgical patients with AC. This study could represent the starting point to push clinical research forward in improving patients’ selection. We observed that the presence of severe comorbidities and AKI are independent predictors of mortality, helping to identify those patients who may benefit from EUS-GBD. We acknowledge that data on predictive factors should be confirmed in larger populations and treatment algorithm should be prospectively validated.
Abbreviations
- AC:
-
Acute cholecystitis
- AKI:
-
Acute kidney injury
- CCI:
-
Charlson Comorbidity Index
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- ET-GBD:
-
Endoscopic transpapillary gallbladder drainage
- EUS:
-
Endoscopic ultrasound
- EUS-GBD:
-
EUS-guided gallbladder drainage
- HR:
-
Hazard ratio
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- LAMS:
-
Lumen-apposing metal stent
- LoS:
-
Length of stay
- MDT:
-
Multidisciplinary team
- OR:
-
Odds ratio
- PT-GBD:
-
Percutaneous transhepatic gallbladder drainage
- ROC:
-
Receiver operating characteristic
- 95% CI:
-
95% confidence intervals
References
European Association for the Study of the Liver (EASL) (2016) EASL clinical practice guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 65:146–181
Buscarini E, Conte D, Cannizzaro R, Bazzoli F, De Boni M, DelleFave G, Farinati F, Ravelli P, Testoni PA, Lisiero M, Spolaore P; Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO), Italian Society of Endoscopy (SIED), Italian Society of Gastroenterology (SIGE) (2014) White paper of Italian Gastroenterology: delivery of services for digestive diseases in Italy: weaknesses and strengths. Dig Liver Dis 46(7):579–589
Brighi N, Lamberti G, Maggio I, Manuzzi L, Ricci C, Casadei R, Santini D, Mosconi C, Lisotti A, Ambrosini V, Pantaleo MA, Campana D (2019) Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig Liver Dis 51:689–694
Brighi N, Panzuto F, Modica R, Gelsomino F, Albertelli M, Pusceddu S, Massironi S, Lamberti G, Rinzivillo M, Faggiano A, Spallanzani A, Ferone D, Prinzi N, Rossi RE, Annibale B, Colao AM, Campana D (2020) Biliary stone disease in patients with neuroendocrine tumors treated with somatostatin analogs: a multicenter study. Oncologist 25:259–265
Keus F, Gooszen HG, van Laarhoven CJ (2010) Open, small-incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato-Biliary Group reviews. Cochrane Database Syst Rev 1:CD008318
Wakabayashi G, Iwashita Y, Hibi T, Takada T, Strasberg SM, Asbun HJ, Endo I, Umezawa A, Asai K, Suzuki K, Mori Y, Okamoto K, Pitt HA, Han HS, Hwang TL, Yoon YS, Yoon DS, Choi IS, Huang WS, Giménez ME, Garden OJ, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Liu KH, Su CH, Misawa T, Nakamura M, Horiguchi A, Tagaya N, Fujioka S, Higuchi R, Shikata S, Noguchi Y, Ukai T, Yokoe M, Cherqui D, Honda G, Sugioka A, de Santibañes E, Supe AN, Tokumura H, Kimura T, Yoshida M, Mayumi T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M (2018) Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary PancreatSci 25:73–86
Okamoto K, Suzuki K, Takada T, Strasberg SM, Asbun HJ, Endo I, Iwashita Y, Hibi T, Pitt HA, Umezawa A, Asai K, Han HS, Hwang TL, Mori Y, Yoon YS, Huang WS, Belli G, Dervenis C, Yokoe M, Kiriyama S, Itoi T, Jagannath P, Garden OJ, Miura F, Nakamura M, Horiguchi A, Wakabayashi G, Cherqui D, de Santibañes E, Shikata S, Noguchi Y, Ukai T, Higuchi R, Wada K, Honda G, Supe AN, Yoshida M, Mayumi T, Gouma DJ, Deziel DJ, Liau KH, Chen MF, Shibao K, Liu KH, Su CH, Chan ACW, Yoon DS, Choi IS, Jonas E, Chen XP, Fan ST, Ker CG, Giménez ME, Kitano S, Inomata M, Hirata K, Inui K, Sumiyama Y, Yamamoto M (2018) Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary PancreatSci 25:55–72
Mori Y, Itoi T, Baron TH, Takada T, Strasberg SM, Pitt HA, Ukai T, Shikata S, Noguchi Y, Teoh AYB, Kim MH, Asbun HJ, Endo I, Yokoe M, Miura F, Okamoto K, Suzuki K, Umezawa A, Iwashita Y, Hibi T, Wakabayashi G, Han HS, Yoon YS, Choi IS, Hwang TL, Chen MF, Garden OJ, Singh H, Liau KH, Huang WS, Gouma DJ, Belli G, Dervenis C, de Santibañes E, Giménez ME, Windsor JA, Lau WY, Cherqui D, Jagannath P, Supe AN, Liu KH, Su CH, Deziel DJ, Chen XP, Fan ST, Ker CG, Jonas E, Padbury R, Mukai S, Honda G, Sugioka A, Asai K, Higuchi R, Wada K, Yoshida M, Mayumi T, Hirata K, Sumiyama Y, Inui K, Yamamoto M (2018) Tokyo Guidelines 2018: management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary PancreatSci 25:87–95
Krishnamoorthi R, Dasari CS, ThoguluvaChandrasekar V, Priyan H, Jayaraj M, Law J, Larsen M, Kozarek R, Ross A, Irani S (2020) EUS-guided versus endoscopic transpapillary gallbladder drainage in high-risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. SurgEndosc 34:1904–1913
Mohan BP, Khan SR, Trakroo S, Ponnada S, Jayaraj M, Asokkumar R, Adler DG (2020) Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy 52:96–106
Fabbri C, Luigiano C, Lisotti A, Cennamo V, Virgilio C, Caletti G, Fusaroli P (2014) Endoscopic ultrasound-guided treatments: are we getting evidence based: a systematic review. World J Gastroenterol 20:8424–8448
Fusaroli P, Jenssen C, Hocke M, Burmester E, Buscarini E, Havre RF, Ignee A, Saftoiu A, Vilmann P, Nolsøe CP, Nürnberg D, D’Onofrio M, Gilja OH, Lorentzen T, Piscaglia F, Sidhu PS, Dietrich CF (2016) EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part v: EUS-Guided Therapeutic Interventions (short version). Ultraschall Med 37:412–420
Teoh AYB, Kitano M, Itoi T, Pérez-Miranda M, Ogura T, Chan SM, Serna-Higuera C, Omoto S, Torres-Yuste R, Tsuichiya T, Wong KT, Leung CH, Chiu PWY, Ng EKW, Lau JYW (2020) Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: an international randomisedmulticentre controlled superiority trial (DRAC 1). Gut 69(6):1085–1091. https://doi.org/10.1136/gutjnl-2019-319996
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J ClinEpidemiol 47:1245–1251
Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, Kozaka K, Endo I, Deziel DJ, Miura F, Okamoto K, Hwang TL, Huang WS, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Noguchi Y, Shikata S, Ukai T, Higuchi R, Gabata T, Mori Y, Iwashita Y, Hibi T, Jagannath P, Jonas E, Liau KH, Dervenis C, Gouma DJ, Cherqui D, Belli G, Garden OJ, Giménez ME, de Santibañes E, Suzuki K, Umezawa A, Supe AN, Pitt HA, Singh H, Chan ACW, Lau WY, Teoh AYB, Honda G, Sugioka A, Asai K, Gomi H, Itoi T, Kiriyama S, Yoshida M, Mayumi T, Matsumura N, Tokumura H, Kitano S, Hirata K, Inui K, Sumiyama Y, Yamamoto M (2018) Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary PancreatSci 25:41–54
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2:1–138
Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ (2010) A lexicon for endoscopic adverse events: report of an ASGE workshop. GastrointestEndosc 71:446–454
Fusaroli P, Serrani M, Lisotti A, D’Ercole MC, Ceroni L, Caletti G (2015) Performance of the forward-view echoendoscope for pancreaticobiliary examination in patients with status post-upper gastrointestinal surgery. Endosc Ultrasound 4:336–341
Lisotti A, Cominardi A, Bacchilega I, Fusaroli P (2019) Failed endoscopic ultrasound-guided gallbladder drainage due to severe bleeding immediately rescued by redo-drainage under contrast-harmonic guidance. Endoscopy 52(6):517–519
Fusaroli P, Serrani M, Sferrazza S, Linguerri R, Jovine E, Lisotti A (2018) Elective cholecystectomy after reversal of septic shock using multimodality endoscopic gallbladder drainage. Endoscopy 50:E299–E300
Luk SW, Irani S, Krishnamoorthi R, Wong Lau JY, Wai Ng EK, Teoh AY (2019) Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy 51:722–732
Fusaroli P, Kypreos D, Alma Petrini CA, Caletti G (2011) Scientific publications in endoscopic ultrasonography: changing trends in the third millennium. J ClinGastroenterol 45:400–404
Fusaroli P, Kypraios D, Eloubeidi MA, Caletti G (2012) Levels of evidence in endoscopic ultrasonography: a systematic review. Dig Dis Sci 57:602–609
Loozen CS, van Santvoort HC, van Duijvendijk P, Besselink MG, Gouma DJ, Nieuwenhuijzen GA, Kelder JC, Donkervoort SC, van Geloven AA, Kruyt PM, Roos D, Kortram K, Kornmann VN, Pronk A, van der Peet DL, Crolla RM, van Ramshorst B, Bollen TL, Boerma D (2018) Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high risk patients (CHOCOLATE): multicentrerandomised clinical trial. BMJ 8(363):k3965. https://doi.org/10.1136/bmj.k3965
Huang SZ, Chen HQ, Liao WX, Zhou WY, Chen JH, Li WC, Zhou H, Liu B, Hu KP (2020) Comparison of emergency cholecystectomy and delayed cholecystectomy after percutaneous transhepatic gallbladder drainage in patients with acute cholecystitis: a systematic review and meta-analysis. Updates Surg. https://doi.org/10.1007/s13304-020-00894-4
Lisotti A, Bacchilega I, Linguerri R, Fusaroli P (2020) Endoscopic ultrasound-guided gallbladder drainage as a strategy to overcome shortage of operating rooms and intensive care unit beds during Covid-19 crisis. Endoscopy 52(7):E263–E264
Lisotti A, Fusaroli P (2020) EUS-guided gallbladder drainage during a pandemic crisis: how the COVID-19 outbreak could impact interventional endoscopy. Dig Liver Dis 52(6):613–614
Funding
The authors received no support or funding for this study.
Author information
Authors and Affiliations
Contributions
AL and PF wrote the manuscript and performed EUS-GBD procedures; AC and GM collected data; IB and RL are responsible for patients' management and revised the manuscript for pivotal intellectual content. All Authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosures
Dr. Andrea Lisotti, Dr. Romano Linguerri, Dr. Igor Bacchilega, Dr. Anna Cominardi, Dr. Gianmarco Marocchi, and Prof. Pietro Fusaroli declare no conflict of interest or any financial relationship with any pharmaceutical or device company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous presentation: ESGE Days 2019 oral presentation, Prague, Saturday, April 6, 2019. Published on Endoscopy 2019; 51(04): S96–S97. https://doi.org/10.1055/s-0039-1681454.
Supplementary Information
Below is the link to the Supplementary Information.
464_2021_8318_MOESM2_ESM.tiff
Supplementary Information 2 (TIFF 71 kb). Supplementary Figure 2 - ROC curve analysis showing the best age-adjusted Charlson Comorbidity Index cut-off point for the prediction of mortality after EUS-GBD
Rights and permissions
About this article
Cite this article
Lisotti, A., Linguerri, R., Bacchilega, I. et al. EUS-guided gallbladder drainage in high-risk surgical patients with acute cholecystitis—procedure outcomes and evaluation of mortality predictors. Surg Endosc 36, 569–578 (2022). https://doi.org/10.1007/s00464-021-08318-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08318-z