Abstract
Background
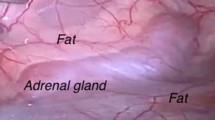
The posterior retroperitoneoscopic adrenal access represents a challenge in orientation and working space creation. The aim of this experimental acute study was to evaluate the impact of computer-assisted quantitative fluorescence imaging on adrenal gland identification and assessment of intraoperative remnant perfusion for adrenal resection in the posterior retroperitoneoscopic approach.
Methods
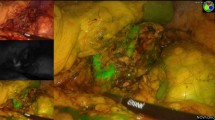
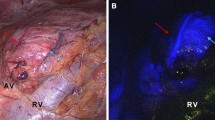
Six pigs underwent simultaneous (n = 5) or sequential (n = 1) bilateral posterior retroperitoneoscopic adrenalectomy (n = 12). Fluorescence imaging was obtained via intravenous administration of 3 mL of Indocyanine Green (ICG) and by switching the camera systems to near-infrared mode (D-LIGHT P, KARL STORZ; Germany). Fluorescence-based visualization of adrenal glands before vascular division (n = 4), after the main vascular pedicle ligation (negative control, n = 1) or after adrenal resection (n = 7), was followed by completion adrenalectomy. The fluorescence signal intensity dynamics were recorded and analyzed using proprietary software. For each pixel, the slope of fluorescence signal intensity evolution over time was translated into a color-coded perfusion cartography, which was superimposed onto real-time images obtained with the corresponding left and right camera systems. Quantitative fluorescence signal analysis in the regions of interest (ROIs) served to assess adrenal remnant perfusion in divided adrenal glands.
Results
In the retroperitoneum, the vascular anatomy was illuminated in fluorescence imaging first. The adrenal glands were promptly highlighted after primary intravenous ICG administration (n = 9) or showed a fluorescence signal intensity increase upon reinjection (n = 3). Quantitative fluorescence analysis showed a statistically significant difference between perfused and ischemic segments in divided glands (p = 0.0156).
Conclusions
Fluorescence imaging provides real-time guidance during minimally invasive adrenal surgery. Prior to dissection, it allows to easily discriminate the adrenal gland from surrounding retroperitoneal structures. After adrenal gland division, ICG injection associated with a computer-assisted quantitative analysis helps to distinguish between well-perfused and ischemic segments. Further studies are underway to establish the correlation between remnant perfusion and viability.





Similar content being viewed by others
References
Weissleder R, Pittet MJ (2008) Imaging in the era of molecular oncology. Nature 452:580–589
Seeliger B, Barberio M, Urso A, Agnus V, Longo F, Mascagni P, Marescaux J, Mutter D, Diana M (2018) Fluorescence in rectal cancer surgery. Ann Laparosc Endosc Surg. https://doi.org/10.21037/ales.22018.21005.21007
Baiocchi GL, Diana M, Boni L (2018) Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: state of the art and future directions. World J Gastroenterol 24:2921–2930
Vrielink OM, Wevers KP, Kist JW, Borel Rinkes IHM, Hemmer PHJ, Vriens MR, de Vries J, Kruijff S (2017) Laparoscopic anterior versus endoscopic posterior approach for adrenalectomy: a shift to a new golden standard? Langenbecks Arch Surg 402:767–773
Gaujoux S, Mihai R, Joint working group of ESES and ENSAT (2017) European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg 104:358–376
Walz MK (1998) Minimally invasive adrenalectomy–comparison of surgical approaches. Langenbecks Arch Chir Suppl Kongressbd 115:113–115
Brunt LM (2013) SAGES guidelines for minimally invasive treatment of adrenal pathology. Surg Endosc 27:3957–3959
Arezzo A, Bullano A, Cochetti G, Cirocchi R, Randolph J, Mearini E, Evangelista A, Ciccone G, Bonjer HJ, Morino M (2018) Transperitoneal versus retroperitoneal laparoscopic adrenalectomy for adrenal tumours in adults. Cochrane Database Syst Rev 12:CD011668
Vrielink OM, Engelsman AF, Hemmer PHJ, de Vries J, Vorselaars W, Vriens MR, Karakatsanis A, Hellman P, Sywak MS, van Leeuwen BL, El Moumni M, Kruijff S (2018) Multicentre study evaluating the surgical learning curve for posterior retroperitoneoscopic adrenalectomy. Br J Surg 105:544–551
Stefanidis D, Goldfarb M, Kercher KW, Hope WW, Richardson W, Fanelli RD, Society of Gastrointestinal and Endoscopic Surgeons (2013) SAGES guidelines for minimally invasive treatment of adrenal pathology. Surg Endosc 27:3960–3980
Perrier ND, Kennamer DL, Bao R, Jimenez C, Grubbs EG, Lee JE, Evans DB (2008) Posterior retroperitoneoscopic adrenalectomy: preferred technique for removal of benign tumors and isolated metastases. Ann Surg 248:666–674
DeLong JC, Chakedis JM, Hosseini A, Kelly KJ, Horgan S, Bouvet M (2015) Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol 112:650–653
Rieder JM, Nisbet AA, Wuerstle MC, Tran VQ, Kwon EO, Chien GW (2010) Differences in left and right laparoscopic adrenalectomy. JSLS 14:369–373
Colvin J, Zaidi N, Berber E (2016) The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J Surg Oncol 114:153–156
Kahramangil B, Berber E (2017) The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol 115:848–855
Pathak RA, Hemal AK (2019) Intraoperative ICG-fluorescence imaging for robotic-assisted urologic surgery: current status and review of literature. Int Urol Nephrol 51:765–771
Diana M, Noll E, Agnus V, Liu YY, Kong SH, Legner A, Diemunsch P, Marescaux J (2017) Reply to letter: “enhanced reality fluorescence videography to assess bowel perfusion: the cybernetic eye”. Ann Surg 265:e49–e52
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259:700–707
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Gene Med 12:561–563
Walz MK, Peitgen K, Diesing D, Petersenn S, Janssen OE, Philipp T, Metz KA, Mann K, Schmid KW, Neumann HP (2004) Partial versus total adrenalectomy by the posterior retroperitoneoscopic approach: early and long-term results of 325 consecutive procedures in primary adrenal neoplasias. World J Surg 28:1323–1329
Walz MK, Alesina PF, Wenger FA, Deligiannis A, Szuczik E, Petersenn S, Ommer A, Groeben H, Peitgen K, Janssen OE, Philipp T, Neumann HP, Schmid KW, Mann K (2006) Posterior retroperitoneoscopic adrenalectomy–results of 560 procedures in 520 patients. Surgery 140:943–948 (Discussion 948–950)
Horta M, Neto N, Couceiro C, Martins L (2014) extraperitoneal space: anatomic and radiologic overview. Eur Congr Radiol. https://doi.org/10.1594/ecr2014/c-2250
Dip FD, Roy M, Perrins S, Ganga RR, Lo Menzo E, Szomstein S, Rosenthal R (2015) Technical description and feasibility of laparoscopic adrenal contouring using fluorescence imaging. Surg Endosc 29:569–574
Dondelinger RF, Ghysels MP, Brisbois D, Donkers E, Snaps FR, Saunders J, Deviere J (1998) Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur Radiol 8:1254–1273
Suzuki K, Nagai H, Hoshino T, Tamate H (1985) The branching site of the posterior adrenal artery in pigs. Okajimas Folia Anat Jpn 62:27–33
Walz MK, Alesina PF (2009) Single access retroperitoneoscopic adrenalectomy (SARA)–one step beyond in endocrine surgery. Langenbecks Arch Surg 394:447–450
Lowery AJ, Seeliger B, Alesina PF, Walz MK (2017) Posterior retroperitoneoscopic adrenal surgery for clinical and subclinical cushing’s syndrome in patients with bilateral adrenal disease. Langenbecks Arch Surg 402:775–785
Alesina PF, Hinrichs J, Meier B, Schmid KW, Neumann HP, Walz MK (2012) Minimally invasive cortical-sparing surgery for bilateral pheochromocytomas. Langenbecks Arch Surg 397:233–238
Brunt LM, Molmenti EP, Kerbl K, Soper NJ, Stone AM, Clayman RV (1993) Retroperitoneal endoscopic adrenalectomy: an experimental study. Surg Laparosc Endosc 3:300–306
Hoenig DM, Magee RR, Chrostek CA, Amaral JF, Stein BS (1995) Direct retroperitoneoscopic adrenalectomy in the porcine model. J Laparoendosc Surg 5:385–388
Park A, Gagner M (1995) A porcine model for laparoscopic adrenalectomy. Surg Endosc 9:807–810
Ludwig AT, Wagner KR, Lowry PS, Papaconstantinou HT, Lairmore TC (2010) Robot-assisted posterior retroperitoneoscopic adrenalectomy. J Endourol 24:1307–1314
Arora E, Bhandarwar A, Wagh A, Gandhi S, Patel C, Gupta S, Talwar G, Agarwal J, Rathore J, Chatnalkar S (2018) Role of indo-cyanine green (ICG) fluorescence in laparoscopic adrenalectomy: a retrospective review of 55 Cases. Surg Endosc 32:4649–4657
Kahramangil B, Kose E, Berber E (2018) Characterization of fluorescence patterns exhibited by different adrenal tumors: determining the indications for indocyanine green use in adrenalectomy. Surgery 164:972–977
Sound S, Okoh AK, Bucak E, Yigitbas H, Dural C, Berber E (2016) Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: a feasibility study. Surg Endosc 30:657–662
Manny TB, Pompeo AS, Hemal AK (2013) Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology 82:738–742
Dobbie JW, Symington T (1966) The human adrenal gland with special reference to the vasculature. J Endocrinol 34:479–489
Walz MK (2004) Extent of adrenalectomy for adrenal neoplasm: cortical sparing (subtotal) versus total adrenalectomy. Surg Clin North Am 84:743–753
Ishizawa T, Saiura A (2019) Fluorescence Imaging for Minimally Invasive Cancer Surgery. Surg Oncol Clin N Am 28:45–60
Quero G, Lapergola A, Barberio M, Seeliger B, Gockel I, Saccomandi P, Guerriero L, Mutter D, Saadi A, Worreth M, Marescaux J, Agnus V, Diana M (2019) Discrimination between arterial and venous bowel ischemia by computer-assisted analysis of the fluorescent signal. Surg Endosc 33:1988–1997
Acknowledgements
The authors would like to thank Catherine Cers-Meunier for illustrating the anatomical features, and Lionel Grienenberger for the video editing. The authors are also grateful to Camille Goustiaux, Christopher Burel, and Guy Temporal, professionals in medical English proofreading, for their assistance in proofreading the manuscript. The laparoscopic equipment for this study has been kindly supplied by KARL STORZ SE & Co. KG.
Funding
This study was funded by a Grant from the ARC Foundation for Cancer Research (9, rue Guy Môquet; 94803 Villejuif Cedex—France, www.fondation-arc.org), within the framework of the ELIOS (Endoscopic Luminescent Imaging for precision Oncologic Surgery) project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Barbara Seeliger, Martin K. Walz, Pier F. Alesina, Vincent Agnus, Raoul Pop, Manuel Barberio, Alend Saadi, and Marc Worreth have no conflicts of interest or financial ties to disclose. Jacques Marescaux is President of both the IRCAD and IHU Institutes, which are partly funded by KARL STORZ, Medtronic, and Siemens Healthcare. Michele Diana is PI of the ELIOS project, which was funded by a grant from the ARC Foundation for Cancer Research (9, rue Guy Môquet; 94803 Villejuif Cedex—France, www.fondation-arc.org).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1—Technique of adrenal localization and perfusion assessment using fluorescence imaging in the simultaneous bilateral retroperitoneoscopic approach. The left adrenal surgery is shown on the left, and the right intervention on the right side. (MP4 336161 kb)
Rights and permissions
About this article
Cite this article
Seeliger, B., Walz, M.K., Alesina, P.F. et al. Fluorescence-enabled assessment of adrenal gland localization and perfusion in posterior retroperitoneoscopic adrenal surgery in a preclinical model. Surg Endosc 34, 1401–1411 (2020). https://doi.org/10.1007/s00464-019-06997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06997-3