Abstract
Background
Liver resection in cirrhotic patients reported to have higher morbidity and mortality rates compared to non-cirrhotic patients. Recently, there is increased acceptance of laparoscopic approach in liver surgery. However, few reports evaluated laparoscopic liver resection (LLR) for hepatocellular carcinoma (HCC) in cirrhotic patients. The aim of this study is to evaluate our experience of LLR for HCC and to compare perioperative and long-term outcomes between patients with and without liver cirrhosis (LC).
Methods
A retrospective analysis of 232 patients who underwent LLR for HCC between 2004 and 2013 was carried out. Patients were divided into two groups according to the pathological status of their liver parenchyma, in terms of presence or absence of LC.
Results
LC group had 141 patients, and non-LC group had 91 patients. There were no statistically significant differences between both groups regarding operation time, blood loss, transfusion requirements, intraoperative complications, hospital stay, and postoperative complications. Long-term oncologic outcomes were comparable between both groups regarding the recurrence rates (p = 0.067), overall survival (OS) rates (p = 0.908), and disease-free survival (DFS) rates (p = 0.197). The 1-, 3-, 5-, and 7-year OS were 91.7, 85.5, 79.4, and 70.1 % in LC group, and 93.9, 86, 79.5, and 72.3 % in non-LC group. The 1-, 3-,5-, and 7-year DFS were 75.3, 52.4, 42.6, and 32.7 % in LC group, and 74.1, 57.6, 55.3, 50.2 % in non-LC group.
Conclusions
LLR for HCC is feasible in patients with LC. Cirrhotic patients showed comparable perioperative and long-term outcomes to non-cirrhotic patients.
Similar content being viewed by others
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide [1, 2]. Liver resection (LR) and transplantation remain the main stay of curative treatment of HCC. However, liver transplantation is restricted by donor shortage, high costs, and the burden of life-long immunosuppression [3, 4]. Therefore, LR remains the commonly used strategy of curative treatment for HCC patients with preserved liver functions. On the other hand, LR in the context of cirrhosis is associated with high incidence of operative difficulties, postoperative complications, and recurrence rates [5, 6].
In the recent years, there is increased acceptance of laparoscopic approach in liver surgery. Indications for laparoscopic liver resection (LLR) are expanded to include malignant tumors, and unfavorable locations as tumors in the postero-superior segments and close to major vessels [7, 8].
Recent studies showed that LLR is associated with reduced operative stress and postoperative complications [9, 10]. Therefore, LLR is expected to be more beneficial to cirrhotic patients. However, it remains a matter of debate whether LLR is appropriate for HCC, especially in cirrhotic patients.
Few reports have evaluated LLR for HCC in cirrhotic patients [11–16]. Most of these reports are limited to sub-capsular tumors located in antero-lateral segments [11–13]. Also, few reported long-term outcomes of LLR for HCC [13, 16].
The aim of this study is to evaluate 10-year single-center experience of LLR for HCC and compare perioperative and long-term outcomes between patients with and without liver cirrhosis.
Methods
Study design
During the period between January 2004 and December 2013, 389 patients underwent liver resection for HCC at Seoul National University Bundang Hospital (SNUBH), South Korea. Of them, 232 patients underwent LLR and were enrolled in this study.
Patients were divided into two groups according to the status of their liver parenchyma, with and without liver cirrhosis (LC). LC group had 141 patients with histologically confirmed F4 cirrhosis according to the Metavir score [17], whereas non-LC group had 91 patients. F3 stage is a pre-cirrhotic liver disease. In this study, we included only patients with overt LC (F4).
Preoperative evaluation
All patients underwent detailed laboratory evaluation including complete blood count, liver function tests, serum α-feto-protein (AFP), blood glucose, and indocyanine green (ICG) clearance. The diagnosis and staging of HCC were done by triphasic computed tomography (CT) of the abdomen. Some patients were also assessed by contrast-enhanced ultrasound (US) and magnetic resonance imaging to detect additional lesions.
For all patients, treatment strategies were discussed at multidisciplinary conferences including hepato-pancreato-biliary surgeon, hepatologists, interventional radiologists, and medical oncologists.
Generally, LR was applied for patients with preserved liver functions (i.e., sufficient future liver remnant), without signs of severe portal hypertension, without evidence of extrahepatic metastasis, and with American Society of Anesthesiologists (ASA) grade <III [18].
Surgical procedure
The types of LRs were defined according to Brisbane 2000 terminology [19]. LRs were classified into minor (≤2 segments) or major (>2 segments) according to Couinaud classification. Anatomical resections were generally more preferred if the future liver remnant is adequate, otherwise non-anatomical resections were applied.
The surgical technique was described elsewhere [8]. Generally, the patient was placed in supine or French position, and a periumbilical port was placed under direct vision. A carbon dioxide pneumo-peritoneum was created with pressure 12 mmHg, and three or four additional ports were used. General abdominal evaluation to exclude metastasis was done, and then liver evaluation was done by intraoperative US to determine tumor location, vascular relations and to exclude multiplicity of tumors. Pringle maneuver was used in some cases for non-anatomical resections. Parenchymal transection was done by combination of ultrasonic shears (harmonic scalpel; Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) and Cavitron ultrasonic surgical aspirator (CUSA; Valleylab, Inc., Boulder, CO, USA). Intraoperative US was routinely used to guide the resection planes. LigaSure (ValleyLab, Inc.) was used to control small vessels, and larger vessels were secured by Hem-o-lock clips (Teleflex Medical, Research Triangle Park, NC, USA). Division of hepatic veins was done by vascular staplers. Fibrin glue sealant (Greenplast, Green Cross Corp., Seoul, Korea) was applied to the cut surface, and the specimen was placed in a retrieval bag and extracted through an enlarged port site or Pfannenstiel incision.
Postoperative care and follow-up
Postoperatively, all patients underwent daily follow-up of liver functions and abdominal CT was done routinely on fifth postoperative day (POD).
After discharge, all patients were followed up every 3 months in the first 2 years and then every 6 months afterward. Follow-up assessments included liver function tests, serum AFP, and triphasic CT scan of the abdomen.
Clinical outcomes
Postoperative morbidity was defined as events occurring during the first 60 PODs and was graded according to the Clavien–Dindo classification [20]. Postoperative biliary fistula and liver cell failure were defined according to the International Study Group of Liver Surgery (ISGLS) [21, 22].
Postoperative mortality was defined as death within 90 days after liver resection. Overall survival (OS) is calculated from the day of surgery to the day of death or last follow-up. Disease-free survival (DFS) is calculated from the day of surgery to the day of tumor recurrence or the day of death or last follow-up.
Statistical analysis
Categorical data are expressed as numbers and percentages, and continuous data are expressed as median and range or mean and standard deviation.
Fisher’s exact test was used to compare categorical variables, and Mann–Whitney test was used to compare continuous variables. Survival rates were calculated by Kaplan–Meier method and were compared by log-rank test.
Data management and statistical analyses were done using IBM-SPSS statistical package (v. 20). A p value <0.05 was considered to be statistically significant.
Results
Baseline characteristics
Baseline characteristics of patients of the two groups are shown in Table 1. There were significant differences between the two groups in liver function tests, platelets count, virology status, and model for end-stage liver disease score, and ICG clearance (15 min). Non-LC group had three Child-Pugh class B patients. These patients were diagnosed as hilar cholangiocarcinoma based on preoperative radiological evaluation, but postoperatively they were diagnosed as HCC invading the bile ducts based on the pathological examination.
Surgical outcomes
Operative data of the two groups are shown in Table 2. There were no statistically significant differences between the two groups regarding tumor number, site, and presence of ascites and lymph nodes. Non-LC group showed larger median tumor size (LC, 2.5 cm; non-LC, 3 cm; p = 0.001).
More minor resections were done in LC group [LC, 124 (97.9 %); non-LC, 71 (78 %); p = 0.011]. There were no statistically significant differences between the two groups regarding operation time, blood loss, transfusion requirements, and intraoperative complications. No operative mortality occurred in both groups.
Open conversion occurred in 13 cases (9.1 %) of LC group and 10 cases of non-LC group (11 %), (p = 0.824). Bleeding was the most common cause of open conversion [LC, 7 (5 %); non-LC, 2 (2.2 %)].
Pathological outcomes
There were no statistically significant differences between groups according to tumor satellites, microvascular invasion, capsular invasion, Edmondson–Steiner grade, or pT stage, as shown in Table 3. The non-LC group had a significantly larger resection margin than did the LC group [LC, 0.8 cm (0.01–6.5); non-LC, 1.3 cm (0.1–6.8); p = 0.019].
Postoperative outcomes
There were no statistically significant differences between groups regarding hospital stay, postoperative complications, complication types, and grades as shown in Table 4.
Fluid collections occurred in 16 patients (11.3 %) in LC group and in six patients (6.6 %) in non-LC. In LC group, they were managed conservatively in four patients (2.8 %), US-guided tube drainage in 11 patients (7.8 %), and surgically in one patient (0.7 %). All cases of fluid collection in the non-LC group were managed using US-guided tube drainage.
Bile leakage occurred in three patients (2.1 %) in LC group which was managed by conservative measures in one patient (0.7 %), US-guided tube drainage in one patient (0.7 %), and endoscopic retrograde biliary stent in one patient (0.7 %). One patient (1.1 %) in non-LC group had bile leakage which was managed by US-guided tube drainage.
Early postoperative mortality occurred in two patients (1.4 %) in LC group due to acute respiratory distress syndrome and multi-organ failure.
Long-term outcomes
The mean follow-up period for all patients was 42.3 ± 29.6 months (39.4 ± 27.4 months in LC group and 44.4 ± 31.6 months in non-LC group). Recurrence occurred in 70 patients (49.6 %) in LC group and 34 patients (37.4 %) in non-LC group; there was no statistically significant difference between the groups (p = 0.067). There were no statistically significant differences between groups in terms of recurrence pattern and recurrence treatment as shown in Table 5.
Mortality occurred in 27 patients (19.1 %) in LC group and 13 patients (14.3 %) in non-LC; there was no statistically significant difference between the two groups (p = 0.238).
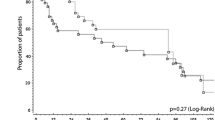
The long-term oncological outcomes were comparable between groups regarding the rates of OS (p = 0.908) and DFS (p = 0.197). The 1-, 3-, 5-, and 7-year OS rates were 91.7, 85.5, 79.4, and 70.1 %, respectively, in the LC group, and 93.9, 86, 79.5, and 72.3 %, respectively, in the non-LC group (Fig. 1). The 1-, 3-, 5-, and 7-year DFS rates were 75.3, 52.4, 42.6, and 32.7 %, respectively, in the LC group, and 74.1, 57.6, 55.3, and 50.2 %, respectively, in the non-LC group (Fig. 2).
Discussion
HCC is associated with LC in approximately 60–90 % of cases, with chronic viral hepatitis (B and C infection) being the main risk factor [23]. Management of HCC that is associated with LC is a complex clinical condition. Selecting the appropriate treatment modality is dependent not only on tumor stage but also on the severity of the underlying liver disease. Nevertheless, LR remains the main curative treatment [24].
When considering LR in the setting of LC, it is important to consider the degree of surgical stress to the patient and the liver, as well as the oncological outcomes. LR in cirrhotic patients is associated with high rates of morbidity and mortality which is partially due to abdominal laparotomy [25].
Recently, LLR has been widely accepted and safely used as a curative treatment for patients with HCC [10]. LLR is associated with several advantages as reduced surgical stress, and blood loss, shorter hospital stay, and decreased postoperative morbidity and mortality. In addition, some studies reported good long-term oncological outcomes in HCC patients [13, 26, 27]. However, it remains unclear whether LLR for HCC is beneficial to cirrhotic patients. Initially, LC was considered a contraindication for LLR [28, 29]. Recently, with the growing surgical experience and introduction of new equipment, some studies reported good perioperative outcomes after LLR for HCC in cirrhotic patients [11, 13, 16, 30]. However, these studies were limited to minor resections of easily accessible lesions and enrolled few numbers of patients. Also, most of them lack the long-term oncological outcomes. The current study included variable-sized tumors located in all liver segments including the postero-superior segments, and all types of liver resections including major hepatectomies, and reported the long-term oncological outcomes.
For long time, there had been a consensus that a diagnosis of Child-Pugh class C was a contraindication for conventional LR [31]. Abdel-Atty et al. [32] reported safe LLR in three Child-Pugh class C patients. In the current study, LLR was performed also in three Child-Pugh class C patients. Although they underwent minor resections, they could recover after surgery without significant morbidities. These findings suggest that laparoscopy enables extension of the indications of LR to include Child-Pugh C patients who are excluded from conventional LR.
In the current study, minor liver resections were more performed in the LC group. In addition, LC group showed a smaller median tumor size and smaller resection margin. As described above, the outcome of LR is dependent not only on tumor stage, but also on the severity of the underlying liver disease [24]. The risk of morbidity after LR depends on the balance between the liver function and the operative procedure used [33]. In our group, we adopted a patient-tailored approach including parenchymal sparing procedures as wedge resection, and anatomical resections in cirrhotic patients with limited hepatic functional reserve [34, 35]. Anatomical LR was performed when the tumor was deeply located or relatively larger in size, whereas a tumorectomy was performed when the tumor was small and located superficially.
Cirrhotic patients have higher morbidity and mortality rates after conventional hepatectomy than do non-cirrhotic patients [36]. Minor open LR in cirrhotic patients is often associated with liver failure, refractory ascites, and wound-related complications [37]. In the current study, there was no statistically significant difference in the incidence of postoperative morbidity and mortality between the two groups. This provides an important finding supporting the safety and the feasibility of LLR for HCC in LC.
Posthepatectomy liver failure (PHLF) and ascites are the primary causes of high early postoperative mortality after LR for HCC [14]. The incidence of PHLF after conventional LR ranges from 1.2 to 32 % [38–41]. LLR is expected to have lower incidence of PHLF and ascites. This may be attributed to less invasiveness of laparoscopy which entails preserved abdominal musculature by avoiding large abdominal incisions and preserved parietal circulation and lymphatics [42, 43]. This advantage could be the most important in the postoperative course after LLR in cirrhotic patients, who could achieve results comparable to non-cirrhotic patients. In the current study, although PHLF and ascites occurred more in LC group, the difference was not statistically significant.
Another important advantage in the postoperative course after LLR in cirrhotic patients is its lower rate of infectious complications. Cirrhotic patients are more vulnerable to such complications because of deterioration of protein synthesis and metabolism, pancytopenia and hypersplenism, and ascites leakage though the wound prevents healing and encounters infection [44]. In the current study, there was no statistically significant difference in the incidence of systemic or local infectious complications between the two groups.
One of the major obstacles of LLR in cirrhotic patients is the risk of massive bleeding. Cirrhotic patients are at great risk of bleeding related to primary hemostasis dysfunction [44]. In the current study, there was no significant difference in the incidence of intraoperative blood loss and transfusion requirements between the two groups. This can be explained by the hemostatic effect of pneumo-peritoneum, better magnification, the use of new devices for parenchymal transection, and the application of anatomical resections. The use of Pringle maneuver in LLR is recommended particularly when performing non-anatomical resections [45, 46].
A high recurrence rate after LR adversely affects the prognosis of HCC patients [47]. Coexisting LC is associated with a higher recurrence rate, possibly due to multicentric carcinogenesis, and lower DFS and OS rates after conventional LR for HCC [48]. In the current study, the recurrence rate was higher in the LC group than in the non-LC group, although the difference was not significant.
In HCC patients, a previous liver resection may compromise subsequent liver transplantation due to postoperative adhesions that increase the surgical difficulty. Such technical obstacles can be reduced by the use of laparoscopy, especially in cirrhotic patients. Laurent et al. [49] compared the outcomes of liver transplantation after initial LLR or OLR. They found that LLR facilitated liver transplantation, in comparison with OLR, in terms of reduced operative time, blood loss, and transfusion requirements. Still there is no data regarding the effect of LLR on liver transplantation regarding tumor recurrence and survival.
Previous studies had identified LC as a negative predictive factor for OS after conventional LR for HCC [44, 50–52]. In the current study, both groups showed similar OS rates up to 7 years after LLR, which is different from other studies reporting conventional LR [44, 50–52]. This advantage could be attributed to the strong follow-up protocol used after LLR, which enables early detection and management of tumor recurrence and thereby improves patient survival (Table 6).
Although the current study included a large number of patients with histologically confirmed liver cirrhosis, it still has some limitations because it was a retrospective and non-randomized study.
In conclusion, the current study demonstrated that LLR for HCC is feasible in patients with cirrhosis. LLR in cirrhotic patients showed comparable results to non-cirrhotic patients in terms of perioperative and long-term outcomes. However, prospective comparative studies are still necessary to prove the superiority of LLR for HCC in cirrhotic patients.
References
Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, Wang L, Tang ZY (2011) Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol 18:1955–1963
Llovet JM, Bruix J (2008) Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 48(Suppl 1):S20–S37
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC (2001) Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430
Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP (2002) Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl 8:873–883
Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R (2000) Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective andnationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 32:1224–1229
Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J (1999) Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 229:210–215
Han HS, Yoon YS, Cho JY, Hwang DW (2013) Laparoscopic liver resection for hepatocellular carcinoma: Korean experiences. Liver Cancer 2:25–30
Han HS, Cho JY, Yoon YS (2009) Techniques for performing laparoscopic liver resection in various hepatic locations. J Hepatobiliary Pancreat Surg 16(4):427–432
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection 2,804 patients. Ann Surg 250:831–841
Parks KR, Kuo YH, Davis JM, O’ Brien B, Hagopian EJ (2014) Laparoscopic versus open liver resection: a meta-analysis of long term outcome. HPB 16(2):109–118
Belli G, Limongelli P, Fantini C, D’Agostino A, Cioffi L, Belli A, Russo G (2009) Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 96:1041–1048
Santambrogio R, Aldrighetti L, Barabino M, Pulitanò C, Costa M, Montorsi M, Ferla G, Opocher E (2009) Laparoscopic liver resections for hepatocellular carcinoma. Is it a feasible option for patients with liver cirrhosis? Langenbecks Arch Surg 394:255–264
Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR (2011) Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc 25(11):3668–3677
Casaccia M, Andorno E, Domenico SD, Nardi I, Bottino G, Gelli M, Valente U (2011) Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients. Feasibility of nonanatomic resection in difficult tumor locations. J Minim Access Surg 7(4):222–226
Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S (2013) Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc 27:2592–2597
Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM (2013) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 257(3):506–511
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293
Wolters U, Wolf T, Stützer H, Schröder T (1996) ASA classification and perioperative variables as predictors of postoperative outcomes. Br J Anaesth 77:217–222
Terminology Committee of the International Hepato-Pancreato-Biliary Association (2000) The IHPBA Brisbane 2000 terminology of liver anatomy and resections. HPB Surg 2:333–339
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149(5):680–688
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 145:713–724
Fattovich G, Stroffolini T, Zagni I, Donato F (2004) Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 127:S35–S50
de Lope CR, Tremosini S, Forner A, Reig M, Bruix J (2012) Management of HCC. J Hepatol 56(Suppl 1):S75–S87
Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D, Fagniez PL (2006) Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with liver disease. Ann Surg 243:499–506
Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I (2010) Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc 24:1170–1176
Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P (2011) Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg 35:2268–2274
Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, Morino M, Bismuth H, Castaing D, Savier E, Honore P, Detry O, Legrand M, Azagra JS, Goergen M, Ceuterick M, Marescaux J, Mutter D, de Hemptinne B, Troisi R, Weerts J, Dallemagne B, Jehaes C, Gelin M, Donckier V, Aerts R, Topal B, Bertrand C, Mansvelt B, Van Krunckelsven L, Herman D, Kint M, Totte E, Schockmel R, Gigot JF (2003) Laparoscopic liver resection of benign liver tumors. Surg Endosc 17:23–30
Marks J, Mouiel J, Katkhouda N, Gugenheim J, Fabiani P (1998) Laparoscopic liver surgery: a report on 28 patients. Surg Endosc 12:331–334
Memeo R, de’Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A (2014) Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case–control study. World J Surg 38:2919–2926
Cauchy F, Fuks D, Belghiti J (2012) HCC: current surgical treatment concepts. Langenbecks Arch Surg 397:681–695
Abdel-Atty MY, Farges O, Jagot P, Belghiti J (1999) Laparoscopy extends the indications for liver resection in patients with cirrhosis. Br J Surg 86(11):1397–1400
Mizuguchi T, Kawamoto M, Meguro M, Nakamura Y, Ota S, Hui TT, Hirata K (2013) Prognosis and predictors of surgical complications in hepatocellular carcinoma patients with or without cirrhosis after hepatectomy. World J Surg 37:1379–1387
Ahn KS, Han HS, Yoon YS, Cho JY, Kim JH (2011) Laparoscopic anatomical S5 segmentectomy by the Glissonian approach. J Laparoendosc Adv Surg Tech A 21(4):345–348
Ahn KS, Han HS, Yoon YS, Cho JY (2011) Laparoscopic anatomic S4 segmentectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 21(4):e183–e186
Ziser A, Plevak DJ, Wiesner RH, Rakela J, Offord KP, Brown DL (1999) Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology 90:42–53
Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O (2000) Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 191:38–46
Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J (1999) Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 229:210–215
Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G (2006) Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl 12:966–971
Dinant S, de Graaf W, Verwer BJ, Bennink RJ, van Lienden KP, Gouma DJ, van Vliet AK, van Gulik TM (2007) Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med 48:685–692
Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN (2007) Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 204:854–862
Aldrighetti L, Guzzetti E, Pulitanò C, Cipriani F, Catena M, Paganelli M, Ferla G (2010) Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 102:82–86
Gaillard M, Tranchart H, Dagher I (2014) Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. WJG 20(17):4892–4899
Cucchetti A, Cescon M, Trevisani F, Pinna AD (2012) Current concepts in hepatic resection for hepatocellular carcinoma in cirrhotic patients. WJG 18(44):6398–6408
Yoon YS, Han HS, Cho JY, Kim JH, Kwon Y (2013) Laparoscopic liver resection for centrally located tumors close to the hilum, major hepatic veins, or inferior vena cava. Surgery 153:502–509
Han HS, Yoon YS, Cho JY, Ahn KS (2010) Laparoscopic right hemihepatectomy for hepatocellular carcinoma. Ann Surg Oncol 17:2090–2091
Takada Y, Otsuka M, Todoroki T, Fukao K (2003) Accompanying liver cirrhosis as a risk factor for recurrence after resection of solitary hepatocellular carcinoma. Hepato-gastroenterol 50(54):1991–1995
Taura K, Ikai I, Hatano E, Yasuchika K, Nakajima A, Tada M, Seo S, Machimoto T, Uemoto S (2007) Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery 142(5):685–694
Laurent A, Tayar C, Andréoletti M, Lauzet JY, Merle JC, Cherqui D (2009) Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 16(3):310–314
Poon RT, Fan ST, Lo CM, Liu CL, Wong J (2002) Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 235:373–382
Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC, Ye SL, Wu ZQ, Fan J, Qin LX, Zheng BH (2001) Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer 91:1479–1486
Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, Ikai I, Yamaoka Y, Curley SA, Nagorney DM, Ng IO, Fan ST, Poon RT, International Cooperative Study Group on Hepatocellular Carcinoma (2005) Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol 12:364–373
Disclosures
Ahmed Shehta, Ho-Seong Han, Yoo-Seok Yoon, Jai Young Cho, and YoungRok Choi declared that there were no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the SAGES 2015 Annual Meeting, April 15–18, 2015, Nashville, Tennessee.
Rights and permissions
About this article
Cite this article
Shehta, A., Han, HS., Yoon, YS. et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: 10-year single-center experience. Surg Endosc 30, 638–648 (2016). https://doi.org/10.1007/s00464-015-4253-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4253-3