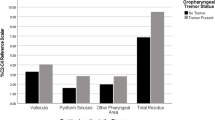
Abstract
The majority of patients with Parkinson’s disease (PD) develop swallowing, speech, and voice (SSV) disorders. Importantly, swallowing difficulty or dysphagia and related aspiration are life-threatening conditions for PD patients. Although PD treatments have significant therapeutic effects on limb motor function, their effects on SSV disorders are less impressive. A large gap in our knowledge is that the mechanisms of SSV disorders in PD are poorly understood. PD was long considered to be a central nervous system disorder caused by the death of dopaminergic neurons in the basal ganglia. Aggregates of phosphorylated α-synuclein (PAS) underlie PD pathology. SSV disorders were thought to be caused by the same dopaminergic problem as those causing impaired limb movement; however, there is little evidence to support this. The pharynx, larynx, and tongue play a critical role in performing upper airway (UA) motor tasks and their dysfunction results in disordered SSV. This review aims to provide an overview on the neuromuscular organization patterns, functions of the UA structures, clinical features of SSV disorders, and gaps in knowledge regarding the pathophysiology underlying SSV disorders in PD, and evidence supporting the hypothesis that SSV disorders in PD could be associated, at least in part, with PAS damage to the peripheral nervous system controlling the UA structures. Determining the presence and distribution of PAS lesions in the pharynx, larynx, and tongue will facilitate the identification of peripheral therapeutic targets and set a foundation for the development of new therapies to treat SSV disorders in PD.



Similar content being viewed by others
Data Availability
The data sources used in this review are publicly available and referenced accordingly in the article. Any additional information or data used in this review can be obtained by contacting the corresponding author.
Abbreviations
- AChE:
-
Acetylcholinesterase
- ASMA:
-
Anti-synuclein monoclonal antibody
- CNS:
-
Central nervous system
- CP:
-
Cricopharyngeus
- CT:
-
Cricothyroid muscle
- ESLN:
-
External superior laryngeal nerve
- GG:
-
Genioglossus
- HG:
-
Hyoglossus
- HN:
-
Hypoglossal nucleus
- IA:
-
Interarytenoid muscle
- IL:
-
Inferior longitudinalis
- IPC:
-
Inferior pharyngeal constrictor
- ISLN:
-
Internal superior laryngeal nerve
- IX:
-
Glossopharyngeal nerve
- IX-L:
-
Lingual branch of the IX nerve
- LCA:
-
Lateral cricoarytenoid muscle
- LN:
-
Lingual nerve
- MPC:
-
Middle pharyngeal constrictor
- NA:
-
Nucleus ambiguous
- PAS:
-
Phosphorylated α-synuclein
- PC:
-
Pharyngeal constrictor
- PCA:
-
Posterior cricoarytenoid muscle
- PD:
-
Parkinson’s disease
- Ph-IX:
-
Pharyngeal branch of the IX nerve
- Ph-X:
-
Pharyngeal branch of the X nerve
- PNS:
-
Peripheral nervous system
- RLN:
-
Recurrent laryngeal nerve
- SG:
-
Styloglossus
- SL:
-
Superior longitudinalis
- SLN:
-
Superior laryngeal nerve
- SPC:
-
Superior pharyngeal constrictor
- SSV:
-
Swallowing,speech and voice
- T:
-
Transversus
- TA:
-
Thyroarytenoid muscle
- UA:
-
Upper airway
- UE:
-
Upper esophagus
- USSLBD:
-
Unified Staging System for Lewy Body Disorders
- V:
-
Verticalis
- VFB:
-
Vocal fold bowing
- X:
-
Vagus nerve
- XII:
-
Hypoglossal nerve
References
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76.
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8(12):1128–39.
Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363(9423):1783–93.
Comi C, Magistrelli L, Oggioni GD, Carecchio M, Fleetwood T, Cantello R, Mancini F, Antonini A. Peripheral nervous system involvement in Parkinson’s disease: evidence and controversies. Parkinsonism Relat Disord. 2014;20(12):1329–34.
Ma C, Zhang W, Cao M. Role of the peripheral nervous system in PD pathology, diagnosis, and treatment. Front Neurosci. 2021;15:598457. https://doi.org/10.3389/fnins.2021.598457.
Del Tredici K, Rub U, De Vos RA, Bohl JRE, Braak H. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61(5):413–26.
Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):2–12.
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–34.
Braak H, Del Tredici K. Invited article: nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70(20):1916–25.
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL 3rd, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702.
Beach TG, Adler CH, Sue LI, Shill HA, Driver-Dunckley E, Mehta SH, et al. Vagus nerve and stomach synucleinopathy in Parkinson’s disease, incidental Lewy body disease, and normal elderly subjects: evidence against the “body-first” hypothesis. J Parkinsons Dis. 2021;11(4):1833–43.
Beach TG, Corbillé AG, Letournel F, Kordower JH, Kremer T, Munoz DG, et al. Multicenter assessment of immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson’s disease and control subjects. J Parkinsons Dis. 2016;6(4):761–70.
Beach TG, Adler CH, Dugger BN, Serrano G, Hidalgo J, Henry-Watson J, et al. Submandibular gland biopsy for the diagnosis of Parkinson disease. J Neuropathol Exp Neurol. 2013;72(2):130–6.
Beach TG, Adler CH, Serrano G, Sue L, Walker DG, Dugger BN, et al. Prevalence of submandibular gland synucleinopathy in Parkinson’s disease, dementia with Lewy bodies and other Lewy body disorders. J Parkinsons Dis. 2016;6(1):153–63.
Beach TG, Carew J, Serrano G, Adler CH, Shill HA, Sue LI, et al. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci Lett. 2014;571:34–8.
Chahine LM, Beach TG, Brumm MC, Adler CH, Coffey CS, Mosovsky S, et al. In vivo distribution of α-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology. 2020;95(9):e1267–e84.
Chahine LM, Beach TG, Adler CH, Hepker M, Kanthasamy A, Appel S, et al. Central and peripheral α-synuclein in Parkinson disease detected by seed amplification assay. Ann Clin Transl Neurol. 2023;10(5):696–705.
Adler CH, Dugger BN, Hinni ML, Lott DG, Driver-Dunckley E, Hidalgo J, et al. Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology. 2014;82(10):858–64.
Adler CH, Dugger BN, Hentz JG, Hinni ML, Lott DG, Driver-Dunckley E, et al. Peripheral synucleinopathy in early Parkinson’s disease: submandibular gland needle biopsy findings. Mov Disord. 2016;31(2):250–6.
Serrano GE, Shprecher D, Callan M, Cutler B, Glass M, Zhang N, et al. Cardiac sympathetic denervation and synucleinopathy in Alzheimer’s disease with brain Lewy body disease. Brain Commun. 2020;2(1):fcaa004.
Manne S, Kondru N, Jin H, Serrano GE, Anantharam V, Kanthasamy A, et al. Blinded RT-QuIC analysis of α-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord. 2020;35(12):2230–9.
Braak H, de Vos RAI, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72.
Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79(6):581–3.
Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N’Guyen JM, et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS ONE. 2010;5(9):e12728.
Cersosimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, et al. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord. 2011;26(1):188–90.
Amino T, Orimo S, Itoh Y, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15(1):29–34.
Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, Takahashi H. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131(Pt 3):642–50.
Dabby R, Djaldetti R, Shahmurov M, Treves TA, Gabai B, Melamed E, Sadeh M, Avinoach I. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm (Vienna). 2006;113(9):1169–76.
Miki Y, Tomiyama M, Ueno T, Haga R, Nishijima H, Suzuki C, et al. Clinical availability of skin biopsy in the diagnosis of Parkinson’s disease. Neurosci Lett. 2010;469(3):357–9.
Lee JM, Derkinderen P, Kordower JH, Freeman R, Munoz DG, Kremer T, et al. The search for a peripheral biopsy indicator of α-synuclein pathology for Parkinson Disease. J Neuropathol Exp Neurol. 2017;76(1):2–15.
Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, et al. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson’s disease. J Neuropathol Exp Neurol. 2013;72(2):119–29.
Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, et al. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013;72(7):614–23.
Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, et al. Altered pharyngeal muscles in Parkinson’s disease. J Neuropathol Exp Neurol. 2012;71(6):520–30.
Mu L, Chen J, Sobotka S, Nyirenda T, Benson B, Gupta F, et al. Alpha-synuclein pathology in sensory nerve terminals of the upper aerodigestive tract of Parkinson’s disease patients. Dysphagia. 2015;30(4):404–17.
Curtis JA, Molfenter SM, Troche MS. Pharyngeal area changes in Parkinson’s disease and its effect on swallowing safety, efficiency, and kinematics. Dysphagia. 2020;35(2):389–98.
Sapir S, Pawlas AA, Ramig LO, et al. Voice and speech abnormalities in Parkinson disease: relation to severity of motor impairment, duration of disease, medication, depression, gender, and age. J Med Speech-Language Pathol. 2001;9:213–26.
Sapir S, Ramig L, Fox C. Voice, speech and swallowing disorders. In: Factor S, Weiner W, editors. Parkinson disease: diagnosis and clinical management. New York: Demos Medical Publishing; 2008. pp. 77–97.
Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson’s disease. Ann Neurol. 1986;19(3):283–7.
Stroudley J, Walsh M. Radiological assessment of dysphagia in Parkinson’s disease. Br J Radiol. 1991;64(766):890–3.
Bird MR, Woodward MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson’s disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Aging. 1994;23(3):251–4.
Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116(2):455–78.
Gorell JM, Johnson CC, Rybicki BA. Parkinson’s disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology. 1994;44(10):1865–8.
Wermuth L, Stenager EN, Stenager E, Boldsen J. Mortality in patients with Parkinson’s disease. Acta Neurol Scand. 1995;92(1):55–8.
Beyer MK, Herlofson K, Arsland D, Larsen JP. Causes of death in a community-based study of Parkinson’s disease. Acta Neurol Scand. 2001;103(1):7–11.
Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8(4):CR241–6.
Mehanna R, Jankovic J. Respiratory problems in neurologic movement disorders. Parkinsonism Relat Disord. 2010;16(10):628–38.
Won JH, Byun SJ, Oh BM, Park SJ, Seo H. Risk and mortality of aspiration pneumonia in Parkinson’s disease: a nationwide database study. Sci Rep. 2021;11:6597.
Hunker CJ, Abbs JH, Barlow SM. The relationship between parkinsonian rigidity and hypokinesia in the orofacial system: a quantitative analysis. Neurology. 1982;32(7):749–54.
Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110(2):383–92.
Hunter PC, Crameri J, Austin S, Woodward MC, Hughes AJ. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry. 1997;63(5):579–83.
Duff J, Sime E. Surgical interventions in the treatment of Parkinson’s disease (PD) and essential tremor (ET): medial pallidotomy in PD and chronic deep brain stimulation (DBS) in PD and ET. Axone. 1997;18(4):85–9.
Kompoliti K, Wang QE, Goetz CG, Leurgans S, Raman R. Effects of central dopaminergic stimulation by apomorphine on speech in Parkinson’s disease. Neurology. 2000;54(2):458–62.
Kent RD, Duffy JR, Slama A, Kent JF, Clift A. Clinicoanatomic studies in dysarthria: review, critique, and directions for research. J Speech Lang Hear Res. 2001;44(3):535–51.
Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Curr Opin Otolaryngol Head Neck Surg. 2008;16(3):205–10.
Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12(1):11–8.
Ertekin C, Tarlaci S, Aydogdu I, Kiylioglu N, Yuceyar N, Turman AB, et al. Electrophysiological evaluation of pharyngeal phase of swallowing in patients with Parkinson’s disease. Mov Disord. 2002;17(5):942–9.
Doty RW. Neural organization of deglutition. In: Code CF, editor. Handbook of physiology. Section 6, Alimentary canal. Volume 4. Washington, D.C.: American Physiological Society; 1968. pp. 1861–902.
Kuna ST, Smickley JS, Vanoye CR. Respiratory-related pharyngeal constrictor muscle activity in normal human adults. Am J Respir Crit Care Med. 1997;155:1991–9.
Minifie FD, Abbs JH, Tarlow A, Kwaterski M. EMG activity within the pharynx during speech production. J Speech Hear Res. 1974;17:497–504.
Standring S, Ellis H, Healy JC, Johnson D, Williams A. Gray’s anatomy. The anatomical basis of clinical practice. 39th ed. New York: Churchill Livingstone; 2005.
Mu L, Sanders I. Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 2007;116(8):604–17.
Sanders I, Mu L. Anatomy of the human internal superior laryngeal nerve. Anat Rec. 1998;252(4):646–56.
Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec. 2000;258(4):406–20.
Pommerenk WT. A study of the sensory areas eliciting the swallowing reflex. Am J Physiol. 1928;84:36–41.
Sanders I, Wu BL, Mu L, Li Y, Biller HF. The innervation of the human larynx. Arch Otolaryngol Head Neck Surg. 1993;119(9):934–9.
Sanders I, Wu BL, Mu L, Biller HF. The innervation of the human posterior cricoarytenoid muscle: evidence for at least two neuromuscular compartments. Laryngoscope. 1994;104(7):880–4.
Mu L, Sanders I, Wu BL, Biller HF. The intramuscular innervation of the human interarytenoid muscle. Laryngoscope. 1994;104(1 Pt 1):33–9.
Mu L, Sanders I. The human cricothyroid muscle: three muscle bellies and their innervation patterns. J Voice. 2009;23(1):21–8.
Storey AT. Laryngeal initiation of swallowing. Exp Neurol. 1968;20(3):359–65.
Shingai T, Shimada K. Reflex swallowing elicited by water and chemical substances applied in the oral cavity, pharynx, and larynx of the rabbit. Jpn J Physiol. 1976;26(5):455–69.
Stedman H, Bradley R, Mistretta C, et al. Chemosensitive responses from the cat epiglottis. Chem Senses. 1980;5:233–45.
Kawasaki A, Fukuda H, Shiotani A, Kanzaki J. Study of movements of individual structures of the larynx during swallowing. Auris Nasus Larynx. 2001;28(1):75–84.
Leopold NA, Kagel MC. Laryngeal deglutition movement in Parkinson’s disease. Neurology. 1997;48(2):373–6.
Hanson DG, Gerratt BR, Ward PH. Cinegraphic observations of laryngeal function in Parkinson’s disease. Laryngoscope. 1984;94(3):348–53.
Smith ME, Ramig LO, Dromey C, Perez KS, Samandari R. Intensive voice treatment in Parkinson disease: laryngostroboscopic findings. J Voice. 1995;9(4):453–9.
Perez KS, Ramig LO, Smith ME, Dromey C. The Parkinson larynx: tremor and videostroboscopic findings. J Voice. 1996;10(4):354–61.
Stelzig Y, Hochhaus W, Gall V, Henneberg A. [Laryngeal manifestations in patients with Parkinson disease]. Laryngorhinootologie. 1999;78(10):544–51.
Blumin JH, Pcolinsky DE, Atkins JP. Laryngeal findings in advanced Parkinson’s disease. Ann Otol Rhinol Laryngol. 2004;113(4):253–8.
Sinclair CF, Gurey LE, Brin MF, Stewart C, Blitzer A. Surgical management of airway dysfunction in Parkinson’s disease compared with Parkinson-plus syndromes. Ann Otol Rhinol Laryngol. 2013;122(5):294–8.
Addington WR, Stephens RE, Gilliland K, Miller SP. Tartaric acid-induced cough and the superior laryngeal nerve evoked potential. Am J Phys Med Rehabil. 1998;77(6):523–6.
Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19(1):44–60.
Miller A. The neuroscientific principles of swallowing and dysphagia. San Diego: Singular Publishing Group, Inc.; 1999.
Bradley RM. Sensory receptors of the larynx. Am J Med. 2000;108(Suppl 4a):47S–50S.
Medda BK, Kern M, Ren J, Xie P, Ulualp SO, Lang IM, Shaker R. Relative contribution of various airway protective mechanisms to prevention of aspiration during swallowing. Am J Gastrointest Liver Physiol. 2003;284(6):G933–9.
Shaker R, Ren J, Bardan E, Eastering C, Dua K, Xie P, Kern M. Pharyngoglottal closure reflex: characterization in healthy young, elderly and dysphagic patients with predeglutitive aspiration. Gerontology. 2003;49(1):12–20.
Murakami Y, Kirchner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59–71.
Nishino T, Tagaito Y, Isono S. Cough and other reflexes on irritation of airway mucosa in man. Pulm Pharmacol. 1996;9(5–6):285–92.
Andreatta RD, Mann EA, Poletto CJ, Ludlow CL. Mucosal afferents mediate laryngeal adductor responses in the cat. J Appl Physiol. 2002;93(5):1622–9.
Meyer TK. The larynx for neurologists. Neurologist. 2009;15(6):313–8.
Addington WR, Stephens RE, Goulding RE. Anesthesia for the superior laryngeal nerves and tartaric acid-induced cough. Arch Phys Med Rehabil. 1999;80(12):1584–6.
Venker-van Haagen AJ, Van den Brom WE, Hellebrekers LJ. Effect of superior laryngeal nerve transection on pharyngeal muscle contraction timing and sequence of activity during eating and stimulation of the nucleus solitarius in dogs. Brain Res Bull. 1999;49(6):393–400.
Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550(Pt 1):287–304.
Luschei ES, Ramig LO, Baker KL, Smith ME. Discharge characteristics of laryngeal single motor units during phonation in young and older adults and in persons with Parkinson disease. J Neurophysiol. 1999;81(5):2131–9.
Merati AL, Heman-Ackah YD, Abaza M, Altman KW, Sulica L, Belamowicz S. Common movement disorders affecting the larynx: a report from the neurolaryngology committee of the AAO-HNS. Otolaryngol Head Neck Surg. 2005;133(5):654–65.
Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297–301.
Niimi A, Matsumoto H, Ueda T, Takemura M, Suzuki K, Tanaka E, et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58(2):152–3.
Aviv JE, Martin JH, Keen MS, Debell M, Blitzer A. Air pulse quantification of supraglottic and pharyngeal sensation: a new technique. Ann Otol Rhinol Laryngol. 1993;102(10):777–80.
Aviv JE. Sensory discrimination in the larynx and hypopharynx. Otolaryngol Head Neck Surg. 1997;116(3):331–4.
Aviv JE, Kim T, Sacco RL, Kaplan S, Goodhart K, Diamond B, Close LG. FEESST: a new bedside endoscopic test of the motor and sensory components of swallowing. Ann Otol Rhinol Laryngol. 1998;107(5 Pt 1):378–87.
Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112(2):338–41.
Hammer MJ, Murphy CA, Abrams TM. Airway somatosensory deficits and dysphagia in Parkinson’s disease. J Parkinsons Dis. 2013;3(1):39–44.
Setzen M, Cohen MA, Mattucci KF, Perlman PW, Ditkoff MK. Laryngopharyngeal sensory deficits as a predictor of aspiration. Otolaryngol Head Neck Surg. 2001;124(6):622–4.
Setzen M, Cohen MA, Perlman PW, Belafsky PC, Guss J, Mattucci KF, Ditkoff M. The association between laryngopharyngeal sensory deficits, pharyngeal motor function, and the prevalence of aspiration with thin liquids. Otolaryngol Head Neck Surg. 2003;128(1):99–102.
Hammer MJ, Barlow SM. Laryngeal somatosensory deficits in Parkinson’s disease: implications for speech respiratory and phonatory control. Exp Brain Res. 2010;201(3):401–9.
He X, Zhang JF, Li ZX, Liu C, Yang LT, Wang N, et al. The traits of five types of tongue movement in Han of Shaanxi, China. Anat Sci Int. 2012;87(4):181–6.
Kappert KDR, van Dijk S, Wellenstein D, van Alphen MJA, van Son RJJH, Smeele LE, Balm AJM. Five specific tongue movements in a healthy population. Dysphagia. 2021;36(4):736–42.
Sanders I, Mu L. A three-dimensional atlas of human tongue muscles. Anat Rec (Hoboken). 2013;296(7):1102–14.
Mu L, Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat. 2010;23(7):777–91.
Saigusa H, Tanuma K, Yamashita K, Saigusa M, Niimi S. Nerve fiber analysis for the lingual nerve of the human adult subjects. Surg Radiol Anat. 2006;28(1):59–65.
Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med. 2001;12(1):18–37.
Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med. 2002;13(5):409–25.
Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Biomechanical basis for lingual muscular deformation during swallowing. Am J Physiol. 1999;277(3):G695–701.
Palmer PM, Jaffe DM, McCulloch TM, Finnegan EM, Van Daele DJ, Luschei ES. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res. 2008;51(4):828–35.
Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14(6):413–29.
Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96(2):440–9.
Tjaden K. Speech and swallowing in Parkinson’s disease. Top Geriatr Rehabil. 2008;24(2):115–26.
Johnson JA, Pring TR. Speech therapy and Parkinson’s disease: a review and further data. Br J Disord Commun. 1990;25(2):183–94.
Brabenec L, Mekyska J, Galaz Z, Rektorova I. Speech disorders in Parkinson’s disease: early diagnostics and effects of medication and brain stimulation. J Neural Transm (Vienna). 2017;124(3):303–34.
Ma EP, Yiu EM. Voice activity and participation profile: assessing the impact of voice disorder on daily activities. J Speech Lang Hear Res. 2001;44(3):511–24.
Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: a review of the literature. J Commun Disord. 2000;33(1):59–88.
Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson’s disease. Lancet Neurol. 2004;3(9):547–56.
Pu T, Huang M, Kong X, Wang M, Chen X, Feng X, Wei C, Weng X, Xu F. Lee Silverman voice treatment to improve speech in Parkinson’s disease: a systemic review and meta-analysis. Parkinsons Dis. 2021;3366870. https://doi.org/10.1155/2021/3366870.
Mancopes R, Smaoui S, Steele CM. Effects of expiratory muscle strength training on videofluoroscopic measures of swallowing: a systematic review. AJSLP. 2020;29:335–56.
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropath. 2009;117(6):613–34.
Adler CH, Beach TG, Zhang N, Shill HA, Driver-Dunckley E, Caviness JN, et al. Unified staging system for Lewy body disorders: clinicopathologic correlations and comparison to Braak staging. J Neuropathol Exp Neurol. 2019;78(10):891–9.
Attems J, Toledo JB, Walker L, Gelpi E, Gentleman S, Halliday G, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-center study. Acta Neuropathol. 2021;141(2):151–72.
Kovari E, Burkhardt K, Lobrinus JA, Bouras C. Lewy body dysphagia. Acta Neuropathol. 2007;114(3):295–8.
Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110(5):517–36.
Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33(6):599–614.
Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci. 2019;12:229.
Acknowledgements
This work was supported by the Department of Defense, Defense Health Program, Congressionally Directed Medical Research Programs (CDMRP), Parkinson’s Research Program under Award No. HT9425-23-1-0481 (to Dr. Liancai Mu). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of human biological materials. The Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30AG019610 and P30AG072980, Arizona Alzheimer’s Disease Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. The authors thank the anonymous reviewers for their constructive comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mu, L., Chen, J., Li, J. et al. Mechanisms of Swallowing, Speech and Voice Disorders in Parkinson’s Disease: Literature Review with Our First Evidence for the Periperal Nervous System Involvement. Dysphagia (2024). https://doi.org/10.1007/s00455-024-10693-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00455-024-10693-3