Abstract
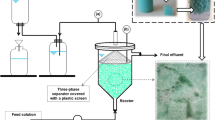
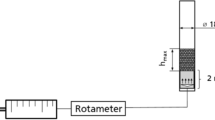
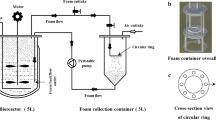
Sophorolipid (SL) production by Candida catenulata from sunflower fatty acids was studied in a bubble column reactor (BCR). The specific oxygen uptake rate was 0.021 mg gcell−1 min−1 which indicates the importance of aeration in SL biosynthesis. The measurement of oxygen transfer rate (OTR) in the BCR showed a satisfactory OTR value of about 0.093 min−1 in the system. However, further SL production was stopped after 30 h in the BCR mainly due to the product accumulation in the culture and its inhibitory effects on cell growth and SL synthesis. Since an extensive foam was generated in the BCR under the absence of an antifoam agent, the development of an in situ foam recovery system provided the integration of production and separation of SL to handle the problem. The application of the foam recovery system enhanced biomass and titer SL concentration by 38.5 and 28.2% in comparison with the conventional BCR, respectively. Further studies in the system were performed by monitoring the size of bubbles and their effects on the biomass and SL enrichment in the foam stream at different aeration rates where the SL enrichment varied from 900 to 100% at 12 and 50 h of the fermentation.








Similar content being viewed by others
Data availability
All research data are reported in the submitted manuscript file.
References
Coutte F, Lecouturier D, Leclère V, Béche M, Jacques P, Dhulster P (2013) New integrated bioprocess for the continuous production, extraction and purification of lipopeptides produced by Bacillus subtilis in membrane bioreactor. Process Biochem 48(1):25–32
de Paula Vieira de Castro R, Rocha VAL, da Silva MECF, Almeida RV, Freire DMG (2022) New insight into the role of oxygen supply for surfactin production in bench‑scale bioreactors using induced surface aeration. Bioprocess Biosyst Eng 45:2031–2041
de Araujo KF, Anna LMMS, Fernandes ACLB, de Menezes RR, Borges CP, Guimarães Freire DMG (2008) Oxygen-controlled biosurfactant production in a bench scale bioreactor. Appl Biochem Biotechnol 147:33–45
Lemlic R (1968) Adsorptive bubble separation methods-foam fractionation and allied techniques. Ind Eng Chem 60(10):16–29
Anic I, Nath A, Franco P, Wichmann R (2017) Foam adsorption as an ex situ capture step for surfactants produced by fermentation. J Biotechnol 258:181–189
Drenckhan W, Saint-Jalmes A (2015) The science of foaming. Adv Colloid Interface Sci 222:228–259
Willenbacher J, Yeremchuk W, Mohr T, Syldatk C, Hausmann R (2015) Enhancement of surfactin yield by improving the medium composition and fermentation process. AMB Express 5:1–9
Davis DA, Lynch HC, Varley J (2001) The application of foaming for the recovery of surfactin from B. subtilis ATCC 21332 cultures. Enzyme Microb Technol 28(4–5):346–354
Willenbacher J, Zwick M, Mohr T, Schmid F, Syldatk C, Hausmann R (2014) Evaluation of different Bacillus strains in respect of their ability to produce surfactin in a model fermentation process with integrated foam fractionation. Appl Microbiol Biotechnol 98:9623–9632
Chen CY, Baker SC, Darton RC (2006) Batch production of biosurfactant with foam fractionation. J Chem Technol Biotechnol 81(12):1923–1931
Sarachat T, Pornsunthorntawee O, Chavadej S, Rujiravanit R (2010) Purification and concentration of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa SP4 using foam fractionation. Bioresour Technol 101(1):324–330
Qazi MA, Wang Q, Dai Z (2022) Sophorolipids bioproduction in the yeast Starmerella bombicola: current trends and perspectives. Bioresour Technol 346:126593
Garg M, Chatterjee M (2018) Isolation characterization and antibacterial effect of biosurfactant from Candida parapsilosis. Biotechnol Rep 18:e00251
Rajasekar V, Darne P, Prabhune A, Kao RYT, Solomon AP, Ramage G (2021) A curcumin-sophorolipid nanocomplex inhibits Candida albicans filamentation and biofilm development. Colloids Surf B 200:111617
De Rienzo MAD, Banat IM, Dolman B, Winterburn J, Martin PJ (2015) Sophorolipid biosurfactants: possible uses as antibacterial and antibiofilm agent. New Biotechnol 32(6):720–726
Gaur VK, Sirohi R, Pandey AK, Varjani S, Pandey A (2022) Sustainable technologies for the production of sophorolipids from renewable wastes. Biomass Biofuels Biochem. Circular Bioeconomy Technol Biofuels Biochem:275–294
Ma X, Meng L, Zhang H, Zhou L, Yue J, Zhu H (2020) Sophorolipid biosynthesis and production from diverse hydrophilic and hydrophobic carbon substrates. Appl Microbiol Biotechnol 104(1):77–100
Zhang Y, Jia D, Sun W, Yang X, Zhang C, Zhao F (2018) Semicontinuous sophorolipid fermentation using a novel bioreactor with dual ventilation pipes and dual sieve-plates coupled with a novel separation system. Microb Biotechnol 11(3):455–464
Dolman BM, Kaisermann C, Martin PJ, Winterburn JB (2017) Integrated sophorolipid production and gravity separation. Process Biochem 54:162–171
Jadhav JV, Pratap AP, Kale SB (2019) Evaluation of sunflower oil refinery waste as feedstock for production of sophorolipid. Process Biochem 78:15–24
Amiri F, Habibi A (2023) Free fatty acids in sunflower oil soap stock progressed the sophorolipid production by Candida catenulata. Can J Chem Eng. https://doi.org/10.1002/cjce.25029
Nourouzpour MM, Habibi A (2023) Degumming process of vegetable oil soap stocks and their applications to biological surfactant production by Candida catenulata. Waste and Biomass Valori. https://doi.org/10.1007/s12649-023-02222-4
Jamali S, Gharaei M, Abbasi S (2016) Identification of yeast species from uncultivated soils by sequence analysis of the hypervariable D1/D2 domain of LSU-rDNA gene in Kermanshah province. Iran Mycologia Iranica 3(2):87–98
Sinegani AAS, Emtiazi G (2006) The relative effects of some elements on the DNS method in cellulase assay. J Appl Sci Environ Manag 10(3):93–96
Sharma MK, Shah DO, Brigham WE (1985) The influence of temperature on surface and microscopic properties of surfactant solutions in relation to fluid displacement efficiency in porous media. AIChE J 31(2):222–228
Kim Y-B, Kim E-K, Lee B-S (2005) Sophorolipid production by Candida bombicola ATCC 22214 from a corn-oil processing byproduct. J Microbiol Biotechnol 15(1):55–58
Ntwampe SKO, Williams CC, Sheldon MS (2010) Water-immiscible dissolved oxygen carriers in combination with Pluronic in bioreactors. Afric J Biotechnol 9(8). https://doi.org/10.5897/AJB09.1007
Alipour S, Habibi A, Taavoni S, Varmira K (2017) β-carotene production from soap stock by loofa-immobilized Rhodotorula rubra in an airlift photobioreactor. Process Biochem 54:9–19
Tharapiwattananon N, Scamehorn JF, Osuwan S, Harwell JH, Haller KJ (1996) Surfactant recovery from water using foam fractionation. Sep Sci Technol 31(9):1233–1258
Stevenson P (2012) Foam engineering: fundamentals and applications. Wiley
Bhakta A, Ruckenstein E (1997) Decay of standing foams: drainage coalescence and collapse. Adv Colloid Interface Sci 70:121–124
Qu Y-H, Zeng G-M, Huang J-H, Xu K, Fang Y-Y, Li X (2008) Recovery of surfactant SDS and Cd2+ from permeate in MEUF using a continuous foam fractionator. J Hazard Mater 155(1–2):32–38
Rubin E, Jorne J (1969) Foam separation of solutions containing two ionic surface-active solutes. Ind Eng Chem Fundam 8(3):474–483
Wu Z, Li N, Zhang X, Xu Y, Shu T, Liu W (2019) Effective recovery of trans-resveratrol from the leaching solution of muscat grape pomace by developing a novel technology of foam fractionation. J Food Eng 241:41–50
Boonyasuwat S, Chavadej S, Malakul P, Scamehorn JF (2005) Surfactant recovery from water using a multistage foam fractionator: Part I effects of air flow rate, foam height, feed flow rate and number of stages. Sep Sci Technol 40(9):1835–1853
Konduru R (1992) Operating a foam fractionating column in simple mode. J Chem Eng Jpn 25(5):548–554
Bikerman JJ (1973) Measurement of foaminess. In: Foams, vol 10. Springer, Berlin, Heidelberg, pp 65–97. https://doi.org/10.1007/978-3-642-86734-7-3
Van Renterghem L, Roelants SLKW, Baccile N, Uyttersprot K, Taelman MC, Everaert B, Mincke S, Ledegen S, Debrouwer S, Scholtens K, Stevens C, Soetaert W (2018) Lab to market: An integrated bioprocess design approach for new-to-nature biosurfactants produced by Starmerella bombicola. Biotechnol Bioeng 115(5):1195–1206
Palme O, Comanescu G, Stoineva I, Radel S, Benes E, Develter D (2010) Sophorolipids from Candida bombicola: cell separation by ultrasonic particle manipulation. Europ J Lipid Sci Technol 112(6):663–673
Anic I, Apolonia I, Franco P, Wichmann R (2018) Production of rhamnolipids by integrated foam adsorption in a bioreactor system. AMB Express 8:1–12
Acknowledgements
The authors would like to thank Kermanshah’s Mahidasht Agricultural Industrial Complex (Nazgol Co., Iran) for giving them sunflower soap stock.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. FA gathered the initial data, managed the literature searches, and produced the initial manuscript. AH anchored the field study, interpreted the data, and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for the review and publication of the manuscript.
Ethics approval
This article does not contain any studies with human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amiri, F., Habibi, A. Handling the inhibitory role of product accumulation during sophorolipid fermentation in a bubble column reactor with an in situ foam recovery. Bioprocess Biosyst Eng 47, 381–392 (2024). https://doi.org/10.1007/s00449-024-02970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-024-02970-0