Abstract
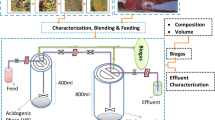
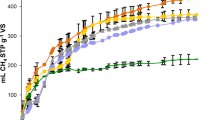
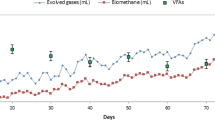
Anaerobic co-digestion (co-AD) of agro-industrial waste, namely, palm oil mill effluent (POME) and sugarcane vinasse (Vn), with water hyacinth (WH) as co-substrate was carried out in two separate Anaerobic Suspended Growth Closed Bioreactors (ASGCBs) under thermophilic (55 °C) conditions. The highest chemical oxygen demand (COD) and soluble COD reduction in co-AD of POME-WH (78.61%, 78.86%) is slightly higher than co-AD of Vn-WH (75.75%, 78.24%). However, VFA reduction in co-AD of POME-WH (96.41%) is higher compared to co-AD of Vn-WH (85.94%). Subsequently, biogas production peaked at 13438 mL/day values and 16122 mL/day for co-AD of POME-WH and Vn-WH, respectively. However, the methane content was higher in the co-AD of POME-WH (72.04%) than in the co-AD of Vn-WH (69.86%). Growth yield (YG), maximum specific substrate utilization rate (rx,max) and maximum specific biomass growth rate (μmax) are higher in co-AD of POME-WH, as supported by the higher mixed liquor volatile suspended solids (MLVSS) and COD reduction efficiency compared to co-AD of Vn-WH. However, methane yield (\({Y}_{{CH}_{4}}\)) reported in the co-AD of POME-WH and Vn-WH are 0.2748 and 0.3112 L CH4/g CODreduction, respectively, which suggests that WH is a more suitable co-substrate for Vn compared to POME.
Graphical Abstract







Similar content being viewed by others
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Chew CL et al (2021) Prospects of palm fruit extraction technology: palm oil recovery processes and quality enhancement. Food Rev Int. https://doi.org/10.1080/87559129.2021.1890117
A. Sri Rahayu et al., Handbook POME to Biogas Project Development in Indonesia, Second edi. In: Castermans, Bernard, Yuwono, Hari, Hardison, Rob, Paramita, Vidia (Eds.), 2015.
Chan YJ, Chong MF (2019) Palm oil mill effluent (POME) treatment—current technologies, biogas capture and challenges. Springer Singapore, Singapore
Nogueira CEC, De Souza SNM, Micuanski VC, Azevedo RL (2015) Exploring possibilities of energy insertion from vinasse biogas in the energy matrix of Paraná State, Brazil. Renew Sustain Energy Rev 48:300–305. https://doi.org/10.1016/j.rser.2015.04.023
Sica P, Carvalho R, Das KC, Baptista AS (2020) Biogas and biofertilizer from vinasse: making sugarcane ethanol even more sustainable. J Mater Cycles Waste Manag 22(5):1427–1433. https://doi.org/10.1007/s10163-020-01029-y
Sydney EB et al (2019) Microalgal biorefineries: Integrated use of liquid and gaseous effluents from bioethanol industry for efficient biomass production. Bioresour Technol 292(August):1–7. https://doi.org/10.1016/j.biortech.2019.121955
Wagh MP, Nemade PD, Sengupta A (2021) Augmentation with Ozone-Assisted Electrochemical degradation of distillery spent wash for the removal of color and chemical oxygen demand. Int J Environ Sci Technol 18(3):619–630. https://doi.org/10.1007/s13762-020-02837-3
Christofoletti CA, Escher JP, Correia JE, Marinho JFU, Fontanetti CS (2013) Sugarcane vinasse: Environmental implications of its use. Waste Manag 33(12):2752–2761. https://doi.org/10.1016/j.wasman.2013.09.005
Karki R et al (2021) Anaerobic co-digestion: current status and perspectives. Bioresour Technol 330:125001. https://doi.org/10.1016/j.biortech.2021.125001
Kumaran P, Hephzibah D, Sivasankari R, Saifuddin N, Shamsuddin AH (2016) A review on industrial scale anaerobic digestion systems deployment in Malaysia: opportunities and challenges. Renew Sustain Energy Rev 56:929–940. https://doi.org/10.1016/j.rser.2015.11.069
Amha YM et al (2018) Inhibition of anaerobic digestion processes: applications of molecular tools. Bioresour Technol 247:999–1014. https://doi.org/10.1016/j.biortech.2017.08.210
Patil JH, AntonyRaj MAL, Shankar BB, Shetty MK, Pradeep Kumar BP (2014) Anaerobic co-digestion of water hyacinth and sheep waste. Energy Procedia 52:572–578. https://doi.org/10.1016/j.egypro.2014.07.112
Henard CA, Smith HK, Guarnieri MT (2017) Phosphoketolase overexpression increases biomass and lipid yield from methane in an obligate methanotrophic biocatalyst. Metab Eng 41:152–158. https://doi.org/10.1016/j.ymben.2017.03.007
Barua VB, Kalamdhad AS (2016) Water hyacinth to biogas: a review. Pollut Res Pap 35:63–73
Venter N, Cowie BW, Witkowski ETF, Snow GC, Byrne MJ (2017) The amphibious invader: rooted water hyacinth’s morphological and physiological strategy to survive stranding and drought events. Aquat Bot 143(September):41–48. https://doi.org/10.1016/j.aquabot.2017.09.004
Wilson JR, Holst N, Rees M (2005) Determinants and patterns of population growth in water hyacinth. Aquat Bot 81(1):51–67. https://doi.org/10.1016/j.aquabot.2004.11.002
Priya P, Nikhitha SO, Anand C, Dipin Nath RS, Krishnakumar B (2018) Biomethanation of water hyacinth biomass. Bioresour Technol 255:288–292. https://doi.org/10.1016/j.biortech.2018.01.119
Feng W et al (2017) Analysis of utilization technologies for Eichhornia crassipes biomass harvested after restoration of wastewater. Bioresour Technol 223:287–295. https://doi.org/10.1016/j.biortech.2016.10.047
Saelor S, Kongjan P, O-Thong S (2017) Biogas production from anaerobic co-digestion of palm oil mill effluent and empty fruit bunches. Energy Procedia 138:717–722. https://doi.org/10.1016/j.egypro.2017.10.206
Bin Khalid Z, Siddique MNI, Nasrullah M, Singh L, Wahid ZBA, Ahmad MF (2019) Application of solar assisted bioreactor for biogas production from palm oil mill effluent co-digested with cattle manure. Environ Technol Innov 16:100446. https://doi.org/10.1016/j.eti.2019.100446
Chan YJ, Lee HW, Selvarajoo A (2021) Comparative study of the synergistic effect of decanter cake (DC) and empty fruit bunch (EFB) as the co-substrates in the anaerobic co-digestion (ACD) of palm oil mill effluent (POME). Environ Chall 5:100257. https://doi.org/10.1016/j.envc.2021.100257
Yap CC et al (2021) Synergistic effect of anaerobic co-digestion of palm oil mill effluent (POME) with Moringa oleifera extract. Biomass Bioenergy 144:105885. https://doi.org/10.1016/j.biombioe.2020.105885
Borges do AV, Fuess LT, Alves I, Takeda PY, Damianovic MHRZ (2021) Co-digesting sugarcane vinasse and distilled glycerol to enhance bioenergy generation in biofuel-producing plants. Energy Convers Manag. https://doi.org/10.1016/j.enconman.2021.114897
Albuquerque JN, Ratusznei SM, Rodrigues JAD (2019) Biomethane production by thermophilic co-digestion of sugarcane vinasse and whey in an AnSBBR: effects of composition, organic load, feed strategy and temperature. J Environ Manage 251:109606. https://doi.org/10.1016/j.jenvman.2019.109606
Sillero L, Solera R, Perez M (2022) Improvement of the anaerobic digestion of sewage sludge by co-digestion with wine vinasse and poultry manure: effect of different hydraulic retention times. Fuel 321:124104. https://doi.org/10.1016/j.fuel.2022.124104
De Siqueira JC, Assemany PP, Alves L, Siniscalchi B (2022) Microbial dynamics and methanogenic potential of co-digestion of sugarcane vinasse and dairy secondary effluent in an upflow anaerobic sludge blanket reactor. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.127654
Seekao N, Sangsri S, Rakmak N, Dechapanya W, Siripatana C (2021) Co-digestion of palm oil mill effluent with chicken manure and crude glycerol: biochemical methane potential by monod kinetics. Heliyon 7(2):e06204. https://doi.org/10.1016/j.heliyon.2021.e06204
Ugwu EI, Américo-Pinheiro JHP, Nwobia LI, Kumar V, Ikechukwu EL, Victor EC (2022) Optimization of parameters in biomethanization process with co-digested poultry wastes and palm oil mill effluents. Clean Chem Eng 3:100033. https://doi.org/10.1016/j.clce.2022.100033
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sustain Energy Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Mendieta O, Castro L, Rodríguez J, Escalante H (2020) Synergistic effect of sugarcane scum as an accelerant co-substrate on anaerobic co-digestion with agricultural crop residues from non-centrifugal cane sugar agribusiness sector. Bioresour Technol 303:122957. https://doi.org/10.1016/j.biortech.2020.122957
Chai A et al (2021) Haldane-Andrews substrate inhibition kinetics for pilot scale thermophilic anaerobic degradation of sugarcane vinasse. Bioresour Technol 336:125319. https://doi.org/10.1016/j.biortech.2021.125319
Chai A et al (2022) Kinetic model discrimination on the biogas production in thermophilic co-digestion of sugarcane vinasse and water hyacinth. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-20251-9
Chai A, Wong YS, Ong SA, Lutpi NA, Sam ST, Kee WC (2022) Effect of operating temperature in the anaerobic degradation of palm oil mill effluent: process performance, microbial community, and biokinetic evaluation. Chem Pap 76(9):5399–5410. https://doi.org/10.1007/s11696-022-02247-4
Wong YS, Teng TT, Ong SA, Morad N, Rafatullah M (2014) Suspended growth kinetic analysis on biogas generation from newly isolated anaerobic bacterial communities for palm oil mill effluent at mesophilic temperature. RSC Adv 4(110):64659–64667. https://doi.org/10.1039/c4ra08483g
APHA, Standard methods for the examination of water and wastewater., 23rd ed. Washington, D.C., 2017.
Da Ros C, Cavinato C, Pavan P, Bolzonella D (2017) Mesophilic and thermophilic anaerobic co-digestion of winery wastewater sludge and wine lees: an integrated approach for sustainable wine production. J Environ Manage. https://doi.org/10.1016/j.jenvman.2016.03.029
Sinbuathong N, Sombat N, Meksumpun S (2019) Comparison of the increase in methane yield using alkali pretreatment for French weed and water lettuce prior to co-digestion. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.13361
Sawanon S, Leungprasert S, Sillapacharoenkul B (2022) ScienceDirect Grass as a high potential by-product: buffalo grass to biogas and the increase of system performance and stability. Int J Hydrogen Energy 47(74):31941–31948. https://doi.org/10.1016/j.ijhydene.2022.01.042
Li L, He Q, Wei Y, He Q, Peng X (2014) Early warning indicators for monitoring the process failure of anaerobic digestion system of food waste. Bioresour Technol 171:491–494. https://doi.org/10.1016/j.biortech.2014.08.089
Liu Y, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci 1125:171–189. https://doi.org/10.1196/annals.1419.019
Lee DH, Behera SK, Kim JW, Park H (2009) Methane production potential of leachate generated from Korean food waste recycling facilities: a lab-scale study. Waste Manag 29(2):876–882. https://doi.org/10.1016/j.wasman.2008.06.033
Zhang P, Chen Y, Zhou Q (2009) Waste activated sludge hydrolysis and short-chain fatty acids accumulation under mesophilic and thermophilic conditions: effect of pH. Water Res 43(15):3735–3742. https://doi.org/10.1016/j.watres.2009.05.036
McCarty PL (1964) Anaerobic waste treatment fundamentals. Public Work 95:91–94
Lin J-G, Chang C-N, Chang S-C (1997) Enhancement of anaerobic digestion of waste activated sludge by alkaline solubilization. Bioresour Technol 62:85–90
Ward AJ, Hobbs PJ, Holliman PJ, Jones DL (2011) Bioresource technology evaluation of near infrared spectroscopy and software sensor methods for determination of total alkalinity in anaerobic digesters. Bioresour Technol 102(5):4083–4090. https://doi.org/10.1016/j.biortech.2010.12.046
Chen G et al (2016) Experimental study of co-digestion of food waste and tall fescue for bio-gas production. Renew Energy 88:273–279. https://doi.org/10.1016/j.renene.2015.11.035
Minale M, Worku T (2014) Anaerobic co-digestion of sanitary wastewater and kitchen solid waste for biogas and fertilizer production under ambient temperature: Waste generated from condominium house. Int J Environ Sci Technol 11(2):509–516. https://doi.org/10.1007/s13762-013-0255-7
Choi HJ (2020) Acid-fermented fish by-products broth: an influence to sludge reduction and biogas production in an anaerobic co-digestion. J Environ Manage 262:110305. https://doi.org/10.1016/j.jenvman.2020.110305
Tsigkou K, Tsafrakidou P, Kopsahelis A, Zagklis D, Zafiri C, Kornaros M (2020) Used disposable nappies and expired food products valorisation through one- & two-stage anaerobic co-digestion. Renew Energy 147:610–619. https://doi.org/10.1016/j.renene.2019.09.028
Latifi P, Karrabi M, Danesh S (2019) Anaerobic co-digestion of poultry slaughterhouse wastes with sewage sludge in batch-mode bioreactors (effect of inoculum-substrate ratio and total solids). Renew Sustain Energy Rev 107(February):288–296. https://doi.org/10.1016/j.rser.2019.03.015
Ratanatamskul C, Manpetch P (2016) Comparative assessment of prototype digester configuration for biogas recovery from anaerobic co-digestion of food waste and rain tree leaf as feedstock. Int Biodeterior Biodegrad 113:367–374. https://doi.org/10.1016/j.ibiod.2016.05.008
Zahedi S, Rivero M, Solera R, Perez M (2018) Mesophilic anaerobic co-digestion of sewage sludge with glycerine: effect of solids retention time. Fuel 215:285–289. https://doi.org/10.1016/j.fuel.2017.11.007
Dai X, Duan N, Dong B, Dai L (2013) High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: Stability and performance. Waste Manag 33(2):308–316. https://doi.org/10.1016/j.wasman.2012.10.018
Zhou Y et al (2011) Influence of substrate-to-inoculum ratio on the batch anaerobic digestion of bean curd refuse-okara under mesophilic conditions. Biomass Bioenerg 35(7):3251–3256
Haider MR, Zeshan S, Yousaf RN, Malik, and C. Visvanathan. (2015) Effect of mixing ratio of food waste and rice husk co-digestion and substrate to inoculum ratio on biogas production. Bioresour Technol 190:451–457. https://doi.org/10.1016/j.biortech.2015.02.105
Zhang W, Xing W, Li R (2018) Real-time recovery strategies for volatile fatty acid-inhibited anaerobic digestion of food waste for methane production. Bioresour Technol 265:82–92. https://doi.org/10.1016/j.biortech.2018.05.098
Zhang W, Li L, Wang X, Xing W, Li R (2020) Role of trace elements in anaerobic digestion of food waste: Process stability, recovery from volatile fatty acid inhibition and microbial community dynamics. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.123796
Mao C, Zhang T, Wang X, Feng Y, Ren G (2017) Process performance and methane production optimizing of anaerobic co-digestion of swine manure and corn straw. Rep Sci. https://doi.org/10.1038/s41598-017-09977-6
Yong Z, Dong Y, Zhang X, Tan T (2015) Anaerobic co-digestion of food waste and straw for biogas production. Renew Energy 78:527–530. https://doi.org/10.1016/j.renene.2015.01.033
Klarosk J et al (2020) Optimization of methane production parameters during anaerobic co- digestion of food waste and garden waste. J Clean Prod 272:123130. https://doi.org/10.1016/j.jclepro.2020.123130
Baldi F, Pecorini I, Iannelli R (2019) Comparison of single-stage and two-stage anaerobic Co-digestion of food waste and activated sludge for hydrogen and methane production. Renew Energy 143(December):1755–1765. https://doi.org/10.1016/j.renene.2019.05.122
Jijai S, Siripatana C, O-Thong S, Ismail N (2016) Kinetic models for prediction of COD effluent from upflow anerobic sludge blanket (UASB) reactor for cannery seafood wastewater. J Teknol Sci Eng 78(5–6):93–99
Darwin Darwin, Fazil Afrizal, Ilham Muhammad, Sarbaini Sarbaini, Purwanto Satria (2017) Kinetics on anaerobic co-digestion of bagasse and digested cow manure with short hydraulic retention time. Res Agric Eng 63(3):121–127. https://doi.org/10.17221/18/2016-RAE
Ahmad A, Ghufran R (2014) Evaluation of the bio-kinetics of cement kiln dust in an upflow anaerobic sludge blanket reactor for treatment of palm oil mill effluent as a function of hydraulic retention time. Sep Purif Technol 133:129–137. https://doi.org/10.1016/j.seppur.2014.06.047
Ning Z, Zhang H, Li W, Zhang R, Liu G, Chen C (2018) Anaerobic digestion of lipid-rich swine slaughterhouse waste: Methane production performance, long-chain fatty acids profile and predominant microorganisms. Bioresour Technol 269:426–433. https://doi.org/10.1016/j.biortech.2018.08.001
Salama ES, Saha S, Kurade MB, Dev S, Chang SW, Jeon BH (2019) Recent trends in anaerobic co-digestion: fat, oil, and grease (FOG) for enhanced biomethanation. Prog Energy Combust Sci 70:22–42. https://doi.org/10.1016/j.pecs.2018.08.002
Usman M, Zha L, Abomohra AEF, Li X, Zhang C, Salama ES (2020) Evaluation of animal- and plant-based lipidic waste in anaerobic digestion: kinetics of long-chain fatty acids degradation. Crit Rev Biotechnol 40(6):733–749. https://doi.org/10.1080/07388551.2020.1756215
Grego A, Mingrone G (1995) Dicarboxylic acids, an alternate fuel substrate in parenteral nutrition: an update. Clin Nutr 14(3):143–148
Elferink SJWHO, Krooneman J, Gottschal JC, Spoelstra SF, Faber F, Driehuis F (2001) Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by lactobacillus buchneri. Appl Environ Microbiol 67(1):125–132. https://doi.org/10.1128/AEM.67.1.125
Pardo I, Ferrer S (2022) Chapter 14 - Malolactic fermentation in white wines. Elsevier, Amsterdam
Farhana A, Lappin SL (2022) Biochemistry, lactate dehydrogenase. StatPearls. StatPearls Publishing, Tampa
Adeva-Andany M et al (2014) Comprehensive review on lactate metabolism in human health. Mitochondrion 17:76–100. https://doi.org/10.1016/j.mito.2014.05.007
Voet D, Voet JG, Pratt CW (2013) Fundamentals of biochemistry: life at the molecular level. Wiley, Hoboken
Wolfe AJ (2005) The acetate switch. Microbiol Mol Biol Rev 69(1):12–50. https://doi.org/10.1128/mmbr.69.1.12-50.2005
Rose IA, Grunberg-Manago M, Korey SR, Ochoa S (1954) Enzymatic phosphorylation of acetate. J Biol Chem 211(2):737–756. https://doi.org/10.1016/s0021-9258(18)71161-7
Acknowledgements
The authors would like to express their sincere gratitude to the Faculty of Civil Engineering Technology Universiti Malaysia Perlis (UniMAP), Fermpro Industries and Malpom Industries.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y-SW, AC; Methodology: Y-SW, AC; Formal analysis and investigation: Y-SW, AC, W-CK; Writing—original draft preparation: AC; Writing—review and editing: Y-SW, S-AO, NAL; Resources: Y-SW, S-AO, NAL, S-TS, H-CK; Supervision: Y-SW, S-AO, NAL, S-TS, TW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chai, A., Wong, YS., Ong, SA. et al. Exploring the potential of thermophilic anaerobic co-digestion between agro-industrial waste and water hyacinth: operational performance, kinetic study and degradation pathway. Bioprocess Biosyst Eng 46, 995–1009 (2023). https://doi.org/10.1007/s00449-023-02879-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02879-0