Abstract
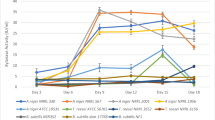
Distillers’ dried grains with solubles (DDGS) is a by-product of dry-mill corn ethanol production comprising a high nutritional value due to residual fiber, protein, and lipid contents. The fiber content of DDGS is high enough to be considered a valuable source for the production of hydrolytic enzymes, such as cellulase and xylanases, which can be used for hydrolysis of lignocellulosic feedstock during ethanol production. The DDGS-based medium prepared after acid hydrolysis provides adequate sugars for enzyme production, while additional macronutrients, such as salts and nitrogen sources, can enhance the enzyme production. Therefore, this study was undertaken to evaluate the effect of salts (KH2PO4, CaCl2·2H2O, MgSO4·7H2O, FeSO4·7H2O, CoCl2·6H2O, and MnSO4·H2O), peptone, and yeast extract on enzyme secretion by four different Aspergillus niger strains and to optimize the nitrogen source for maximum enzyme production. Yeast extract improved the cellulase production (0.38 IU/ml) for A. niger (NRRL 1956) as compared to peptone (0.29 IU/ml). However, maximum cellulase productions of 0.42 IU/ml and 0.45 IU/ml were obtained by A. niger (NRRL 330) and A. niger (NRRL 567), respectively, in presence of ammonium sulfate. The optimized nitrogen amounts resulted in a significant increase in the cellulase production from 0.174 to 0.63 IU/ml on day 9 of the fermentation with A. niger (NRRL 330). The composite model improved both cellulase and xylanase production. In conclusion, the optimization of all three nitrogen sources improved both cellulase and xylanase production in the DDGS-based media.







Similar content being viewed by others
References
RFA (2019) 2019 ethanol industry outlook. https://ethanolrfa.org/wp-content/uploads/2019/02/RFA2019Outlook.pdf. Accessed 26 Dec 2019
RFA (2019) Annual world fuel ethanol production. https://ethanolrfa.org/statistics/annual-ethanol-production/. Accessed 26 Dec 2019
Liu K (2011) Chemical composition of distillers grains, a review. J Agric Food Chem 59:1508–1526
Iram A, Cekmecelioglu D, Demirci A (2020) Distillers’ dried grains with solubles (DDGS) and its potential as the fermentation feedstock. Appl Microbiol Biotechnol (in review)
Świątkiewicz S, Koreleski J (2008) The use of distillers dried grains with solubles (DDGS) in poultry nutrition. Worlds Poult Sci J 64:257–266
Cromwell GL, Herkelman KL, Stahly TS (1993) Physical, chemical, and nutritional characteristics of distillers dried grains with solubles for chicks and pigs. J Anim Sci 71:679–686
Nghiem NP, Montanti J, Kim TH (2016) Pretreatment of dried distiller grains with solubles by soaking in aqueous ammonia and subsequent enzymatic/dilute acid hydrolysis to produce fermentable sugars. Appl Biochem Biotechnol 179:237–250
Houweling-Tan GBN, Sperber BLHM, van der Wal H et al (2016) Barley distillers dried grains with solubles (DDGS) as feedstock for production of acetone, butanol and ethanol. Wageningen University, Wageningen
Brock S, Kuenz A, Prüße U (2019) Impact of hydrolysis methods on the utilization of agricultural residues as nutrient source for d-lactic acid production by Sporolactobacillus inulinus. Fermentation 5:12
Kang Q, Appels L, Tan T, Dewil R (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. Sci World J 2014:13
Zhao X-Q, Zi L-H, Bai F-W et al (2011) Bioethanol from lignocellulosic biomass. biotechnology in China III: biofuels and bioenergy. Springer, Berlin, pp 25–51
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52:858–875
Graham-Rowe D (2011) Beyond food versus fuel. Nature 474:S6
Chrenková M, Čerešňáková Z, Formelová Z et al (2012) Chemical and nutritional characteristics of different types of DDGS for ruminants. J Anim Feed Sci 663:127
Cekmecelioglu D, Demirci A (2020) Production of cellulase and xylanase enzymes using distillers dried grains with solubles (DDGS) by Trichoderma reesei at shake-flask scale and the validation in the benchtop scale bioreactor. Waste Biomass Valoriz 1–10 (in press)
Ximenes EA, Dien BS, Ladisch MR et al (2007) Enzyme production by industrially relevant fungi cultured on coproduct from corn dry grind ethanol plants. In: Mielenz JR, Klasson KT, Adney WS, McMillan JD (eds) Applied biochemistry and biotechnology. ABAB symposium. Humana Press, Totowa, pp 171–183. https://doi.org/10.1007/978-1-60327-181-3_16
Prajapati AS, Panchal KJ, Pawar VA et al (2018) Review on cellulase and xylanase engineering for biofuel production. Ind Biotechnol 14:38–44
Ghori MI, Ahmed S, Malana MA, Jamil A (2011) Corn stover-enhanced cellulase production by Aspergillus niger NRRL 567. Afr J Biotechnol 10:5878–5886
Peterson R, Nevalainen H (2012) Trichoderma reesei RUT-C30–thirty years of strain improvement. Microbiology 158:58–68
Maki M, Leung KT, Qin W (2009) The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci 5:500–516a
Iram A, Cekmecelioglu D, Demirci A (2020) Screening of bacterial and fungal strains for cellulase and xylanase production using distillers’ dried grains with solubles (DDGS) as the main feedstock. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-019-00588-x
Ahamed A, Vermette P (2008) Enhanced enzyme production from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger LMA grown as fed batch in a stirred tank bioreactor. Biochem Eng J 42:41–46. https://doi.org/10.1016/j.bej.2008.05.007
Ahamed A, Vermette P (2008) Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem Eng J 40:399–407
Rodriguez-Gomez D, Hobley TJ (2013) Is an organic nitrogen source needed for cellulase production by Trichoderma reesei Rut-C30? World J Microbiol Biotechnol 29:2157–2165
Clarke KG (2013) Bioprocess engineering: an introductory engineering and life science approach. Elsevier, Amsterdam
Izmirlioglu G, Demirci A (2015) Enhanced bio-ethanol production from industrial potato waste by statistical medium optimization. Int J Mol Sci 16:24490–24505
Hao X-C, Yu X-B, Yan Z-L (2006) Optimization of the medium for the production of cellulase by the mutant Trichoderma reesei WX-112 using response surface methodology. Food Technol Biotechnol 44:89–94
Ellilä S, Fonseca L, Uchima C et al (2017) Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol Biofuels 10:30
Iram A, Cekmecelioglu D, Demirci A (2019) Optimization of dilute sulfuric acid, aqueous ammonia, and steam explosion as the pretreatments steps for distillers’ dried grains with solubles as a potential fermentation feedstock. Bioresour Technol 282:475–481
Ahamed A, Vermette P (2009) Effect of culture medium composition on Trichoderma reesei’s morphology and cellulase production. Bioresour Technol 100:5979–5987
Germec M, Turhan I (2019) Evaluation of carbon sources for the production of inulinase by Aspergillus niger A42 and its characterization. Bioprocess Biosyst Eng 42:1993–2005
Ghose TK, Bisaria VS (1987) Measurement of hemicellulase activities: part I xylanases. Pure Appl Chem 59:1739–1751
Spiehs MJ, Whitney MH, Shurson GC (2002) Nutrient database for distiller’s dried grains with solubles produced from new ethanol plants in Minnesota and South Dakota. J Anim Sci 80:2639–2645
Nuez Ortín WG, Yu P (2009) Nutrient variation and availability of wheat DDGS, corn DDGS and blend DDGS from bioethanol plants. J Sci Food Agric 89:1754–1761
Cheilas T, Stoupis T, Christakopoulos P et al (2000) Hemicellulolytic activity of Fusarium oxysporum grown on sugar beet pulp. Production of extracellular arabinanase. Process Biochem 35:557–561
Godden B, Legon T, HelvensteinPenninckx PM (1989) Regulation of the production of hemicellulolytic and cellulolytic enzymes by a Streptomyces sp. growing on lignocellulose. Microbiology 135:285–292
Ashokkumar B, Kayalvizhi N, Gunasekaran P (2001) Optimization of media for β-fructofuranosidase production by Aspergillus niger in submerged and solid state fermentation. Process Biochem 37:331–338
Barton LL, Georgi CE, Lineback DR (1972) Effect of maltose on glucoamylase formation by Aspergillus niger. J Bacteriol 111:771–777
Izmirlioglu G, Demirci A (2016) Strain selection and medium optimization for glucoamylase production from industrial potato waste by Aspergillus niger. J Sci Food Agric 96:2788–2795
Acharya PB, Acharya DK, Modi HA (2008) Optimization for cellulase production by Aspergillus niger using saw dust as substrate. Afr J Biotechnol 7:4147–4152
Barrington S, Kim J-W (2008) Response surface optimization of medium components for citric acid production by Aspergillus niger NRRL 567 grown in peat moss. Bioresour Technol 99:368–377
Teugjas H, Väljamäe P (2013) Selecting β-glucosidases to support cellulases in cellulose saccharification. Biotechnol Biofuels 6:105
Hansson L, Dostálek M (1988) Effect of culture conditions on mycelial growth and production of γ-linolenic acid by the fungus Mortierella ramanniana. Appl Microbiol Biotechnol 28:240–246
Teugjas H, Väljamäe P (2013) Product inhibition of cellulases studied with 14 C-labeled cellulose substrates. Biotechnol Biofuels 6:104
Dhillon GS, Kaur S, Brar SK, Verma M (2012) Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind Crops Prod 38:6–13
Funding
This study was funded in-part by FULBRIGHT Student Program and USDA National Institute of Food and Agriculture Federal Appropriations under Project PEN04671 and accession number 1017582.
Author information
Authors and Affiliations
Contributions
AD and DC conceived the idea. AI conducted the research, analyzed the data and drafted the manuscript. AD and DC critically evaluated and made significant improvements. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Attia Iram declares that she has no conflict of interest. Deniz Cekmecelioglu declares that he has no conflict of interest. Ali Demirci declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iram, A., Cekmecelioglu, D. & Demirci, A. Salt and nitrogen amendment and optimization for cellulase and xylanase production using dilute acid hydrolysate of distillers’ dried grains with solubles (DDGS) as the feedstock. Bioprocess Biosyst Eng 45, 527–540 (2022). https://doi.org/10.1007/s00449-021-02676-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02676-7