Abstract
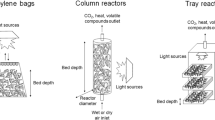
The use of Metarhizium anisopliae as a bioinsecticide is steeply increasing worldwide. However, to reduce the production costs, it is necessary to develop sophisticated techniques for conidia production. This work aimed to use a tray bioreactor to produce conidia of M. anisopliae ICB-425 in long rice and find the limiting bed depth in which the production is still viable. Experiments have been carried out to assess the influence of the air temperature and relative humidity on the spore concentration in order to determine the limiting temperature. Two scales of bioreactors in plastic packages have been used, containing 10 and 500 g of rice, and the results were similar. In the tray bioreactor, the bed depths of 2, 4 and 6 cm have been used, corresponding to the dry rice weights of 1, 2 and 3 kg, respectively, and the results were similar to the ones in plastic packages. A one-phase heat transfer model has been used to foresee the maximum temperature within the bed and the results agreed fairly well with the experimental ones. Using the model, a bed depth of 7 cm was found to be the limit for the tray bioreactor. The results obtained are very promising for the mass production of conidia of M. anisopliae at lower costs and with more effective control.







Similar content being viewed by others
References
Nishi O, Sato H (2017) Species diversity of the entomopathogenic fungi Metarhizium anisopliae and M. flavoviride species complexes isolated from insects in Japan. Mycoscience 58:472–479. https://doi.org/10.1016/j.myc.2017.06.008
Loureiro ES, Batista Filho A, Almeida JEM, Mendes JM, Pessoa LGA (2012) Efficiency of Metarhizium anisopliae (Metsch.) Sorok isolates in the control of the sugarcane spittlebug Mahanarva fimbriolata (Stal, 1854) (Hemiptera: Cercopidae) in field conditions. Arq Inst Biol 79:47–53. https://doi.org/10.1590/S1808-16572012000100007
Castro T, Mayerhofer J, Enkerli J, Eilenberg J, Meyling NV, Moral RA, Demétrio CGB, Delalibera I (2016) Persistence of Brazilian isolates of the entomopathogenic fungi Metarhizium anisopliae and M. robertsii in strawberry crop soil after soil drench application. Agric Ecosyst Environ 233:361–369. https://doi.org/10.1016/j.agee.2016.09.031
lonso-Díaz MA, García L, Galindo-Velasco E, Lezama-Gutierrez R, Angel-Sahagún CA, Rodríguez-Vivas RI, Fragoso-Sánchez H (2007) Evaluation of Metarhizium anisopliae (Hyphomycetes) for the control of Boophilus microplus (Acari: Ixodidae) on naturally infested cattle in the Mexican tropics. Vet Parasitol 147:336–340. https://doi.org/10.1016/j.vetpar.2007.03.030
Paula A, Frazzon G, Da I, Vaz Junior S, Masuda A, Schrank A, Vainstein MH (2000) In vitro assessment of Metarhizium anisopliae isolates to control the cattle tick Boophilus microplus. Vet Parasitol 94:117–125. https://doi.org/10.1016/S0304-4017(00)00368-X
Polar P, De Muro MA, Kairo MTK, Moore D, Pegram R, John SA, Roach-Benn C (2005) Thermal characteristics of Metarhizium anisopliae isolates important for the development of biological pesticides for the control of cattle ticks. Vet Parasitol 134:159–167. https://doi.org/10.1016/j.vetpar.2005.07.010
Galindo-Velasco E, Lezama-Gutiérrez R, Cruz-Vázquez C, Pescador-Rubio A, Angel-Sahagún CA, Ojeda-Chi MM, Rodríguez-Vivas RI, Contreras-Lara D (2015) Efficacy of entomopathogenic fungi (Ascomycetes: Hypocreales) against adult Haematobia irritans (Diptera: Muscidae) under stable conditions in the Mexican dry tropics. Vet Parasitol 209:173–178. https://doi.org/10.1016/j.vetpar.2015.02.025
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41. https://doi.org/10.1016/j.jip.2015.07.009
Faria MR, Wraight SP (2007) Mycoinsecticides and Mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43:237–256. https://doi.org/10.1016/j.biocontrol.2007.08.001
Kruger RD, Posadas JB, Lewylle MA, Mini JI, Lecuona RE (2014) Solid substrate production and formulation of an isolate of Metarhizium anisopliae for biological control of stem bug Tibraca limbativentrisa. World Appl Sci J 32:1242–1251. https://doi.org/10.5829/idosi.wasj.2014.32.07.82407
Santos V, Mascarin GM, da Silva Lopes M, Alves MCDF, Rezende JM, Gatti MSV, Dunlap CA, Delalibera Júnior Í (2017) Identification of double-stranded RNA viruses in Brazilian strains of Metarhizium anisopliae and their effects on fungal biology and virulence. Plant Gene 11:49–58. https://doi.org/10.1016/j.plgene.2017.01.001
Schmidt F GV, Silva JBT, Faria MR, Magalhães BP, Alves RT, Lecoq M (2007) Methodology of application of the fungus Metarhizium anisopliae var. acridum for the control of the grasshopper Rhammatocerus schistocercoides in the field. In: Bol Pesq Desenvolv. 208. https://www.embrapa.br/web/mobile/publicacoes/-/publicacao/189419/metodologia-de-aplicacao-do-fungo-metarhizium-anisopliae-var-acridum-para-o-controle-do-gafanhoto-rhammatocerus-schistocercoides-em-campo
Dorta B, Arcas J (1998) Sporulation of Metarhizium anisopliae in solid-state fermentation with forced aeration. Enzyme Microb Technol 23:501–505. https://doi.org/10.1016/S0141-0229(98)00079-9
Méndez-González F, Loera-Corral O, Saucedo-Castañeda G, Favela-Torres E (2018) Chapter 7: Bioreactors for the production of biological control agents produced by solid-state fermentation. Curr Dev Biotechnol Bioeng 7:109–121. https://doi.org/10.1016/b978-0-444-63990-5.00007-4
Van Breukelen FR, Haemers S, Wijffels RH, Rinzema A (2011) Bioreactor and substrate selection for solid-state cultivation of the malaria mosquito control agent Metarhizium anisopliae. Process Biochem 46:751–757. https://doi.org/10.1016/j.procbio.2010.11.023
Arzumanov T, Jenkins N, Roussos S (2005) Effect of aeration and substrate moisture content on sporulation of Metarhizium anisopliae var. acridum. Process Biochem 40:1037–1042. https://doi.org/10.1016/j.procbio.2004.03.013
Casciatori FP, Bück A, Thoméo JC, Tsotsas E (2016) Two-phase and two-dimensional model describing heat and water transfer during solid-state fermentation within a packed-bed bioreactor. Chem Eng J 287:103–116. https://doi.org/10.1016/j.cej.2015.10.108
Mitchell DA, de Lima Luz LF, Krieger N, Berovič M (2011) Bioreactors for solid-state fermentation. Compr Biotechnol Second Ed 2:347–360. https://doi.org/10.1016/B978-0-08-088504-9.00107-0
Mitchell DA, Von Meien OF, Krieger N (2003) Recent developments in modeling of solid-state fermentation: heat and mass transfer in bioreactors. Biochem Eng J 13:137–147. https://doi.org/10.1016/S1369-703X(02)00126-2
Arora S, Rani R, Ghosh S (2018) Bioreactors in solid state fermentation technology: design, applications and engineering aspects. J Biotechnol 269:16–34
Ye SD, Ying SH, Chen C, Feng MG (2006) New solid-state fermentation chamber for bulk production of aerial conidia of fungal biocontrol agents on rice. Biotechnol Lett 28:799–804. https://doi.org/10.1007/s10529-006-9004-z
Rajagopalan S, Modak JM (1994) Heat and mass transfer simulation studies for solid-state fermentation processes. Chem Eng Sci 49:2187–2193. https://doi.org/10.1016/0009-2509(94)E0012-F
Perry RH, Benskow LR, Beimesch WE, Hecht JP, Kemp I, Langrish T, Schwartzbach C, Smith FL (2008) Perry’s chemical engineers’ handbook, 8th edn. McGraw-Hil, New York
Choi YOMR (1986) Effects of temperature and composition on thermal properties of food. Food engineering and process applications. In: Le Maguer M, Jelen P (eds) Transport phenomenon. Elsevier, London, pp 93–101
Doran MD (1995) Bioprocess engineering principles. Elsevier Trade Monographs, London
Sangsurasak P, Mitchell DA (1998) Validation of a model describing two-dimensional heat transfer during solid-state fermentation in packed bed bioreactors. Biotechnol Bioeng 60:739–748. https://doi.org/10.1002/(SICI)1097-0290(19981220)60:6%3c739:AID-BIT10%3e3.0.CO;2-U
Walstad J, Anderson R, Stambaugh W (1970) Effects of environmental conditions on two species of muscardine fungi (Beauveria bassiana and Metarhizium anisopliae). J Invertebr Pathol 16:221–226
Arthurs S, Thomas MB (2001) Effects of temperature and relative humidity on sporulation of Metarhizium anisopliae var. acridum in mycosed cadavers of Schistocerca gregaria. J Invertebr Pathol 78:59–65. https://doi.org/10.1006/jipa.2001.5050
Hallsworth JE, Magan N (1999) Water and temperature relations of growth of the entomogenous fungi Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. J Invertebr Pathol 74:261–266. https://doi.org/10.1006/jipa.1999.4883
Keyser CA, Fernandes ÉKK, Rangel DEN, Roberts DW (2014) Heat-induced post-stress growth delay: a biological trait of many Metarhizium isolates reducing biocontrol efficacy? J Invertebr Pathol 120:67–73. https://doi.org/10.1016/j.jip.2014.05.008
Li J, Feng MG (2009) Intraspecific tolerance of Metarhizium anisopliae conidia to the upper thermal limits of summer with a description of a quantitative assay system. Mycol Res 113:93–99. https://doi.org/10.1016/j.mycres.2008.08.006
Lanza LM, Monteiro AC, Malheiros EB (2009) Sensibilidade de Metarhizium anisopliae à temperatura e umidade em três tipos de solos. Ciênc Rural 39:6–12. https://doi.org/10.1590/S0103-84782009000100002
Inglis G, Goettel M, Butt T, Strasser H (2001) Use of hyphomycetous fungi for managing insects pest. In: Butttm TM et al (eds) Fungi as biocontrol agents progress, problems and potential. CABI, Wallingford, pp 23–70
Wraight SP, Jackson MA, de Kokc SL (2001) Production, stabilization and formulation of fungal biocontrol agents. Fungi as biocontrol agents: progress, problems and potential. CABI, Wallingford, pp 253–287
Mitchell DA, Pandey A, Sangsurasak P, Krieger N (1999) Scale-up strategies for packed-bed bioreactors for solid-state fermentation. Process Biochem 35:167–178
Farinas CS, Vitcosque GL, Fonseca RF, Neto VB, Couri S (2011) Modeling the effects of solid state fermentation operating conditions on endoglucanase production using an instrumented bioreactor. Ind Crops Prod 34:1186–1192. https://doi.org/10.1016/j.indcrop.2011.04.006
Rajagopalan S, Modak JM (1995) Modelling of heat and mass transfer for solid state fermentation process in tray bioreactor. Bioprocess Eng 13:161–169. https://doi.org/10.1007/BF00369700
Szewczyk KW, Myszka L (1994) The effect of temperature on the growth of A. niger in solid state fermentation. Bioprocess Eng 10:123–126. https://doi.org/10.1007/BF00369467
Smits JP, Van Sonsbeek HM, Knol W, Tramper J, Geelhoed W, Peeters M, Rinzema A (1999) Modelling fungal solid-state fermentation: the role of inactivation kinetics. Bioprocess Eng 20:391–404. https://doi.org/10.1007/s004490050607
Acknowledgements
The authors would like to thank to the São Paulo Research Foundation (FAPESP, Proc. 2006/55641-7, 2018/00996-2) for the financial support and CAPES for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lucas Portilho da Cunha, Fernanda Perpétua Casciatori, Isabella de Cenço Lopes and João Cláudio Thoméo declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Cunha, L.P., Casciatori, F.P., de Cenço Lopes, I. et al. Production of conidia of the entomopathogenic fungus Metarhizium anisopliae ICB 425 in a tray bioreactor. Bioprocess Biosyst Eng 42, 1757–1768 (2019). https://doi.org/10.1007/s00449-019-02172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02172-z