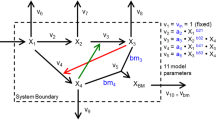
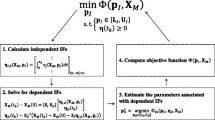
Abstract
Dynamic flux balance analysis (DFBA) has become an instrumental modeling tool for describing the dynamic behavior of bioprocesses. DFBA involves the maximization of a biologically meaningful objective subject to kinetic constraints on the rate of consumption/production of metabolites. In this paper, we propose a systematic data-based approach for finding both the biological objective function and a minimum set of active constraints necessary for matching the model predictions to the experimental data. The proposed algorithm accounts for the errors in the experiments and eliminates the need for ad hoc choices of objective function and constraints as done in previous studies. The method is illustrated for two cases: (1) for in silico (simulated) data generated by a mathematical model for Escherichia coli and (2) for actual experimental data collected from the batch fermentation of Bordetella Pertussis (whooping cough).






Similar content being viewed by others
Abbreviations
- S:
-
Stoichiometric matrix
- \({{\varvec{v}}_{\varvec{k}}}\) :
-
Vector of fluxes
- \(n\) :
-
Number of reactions
- \(k\) :
-
Time instance
- \(\psi\) :
-
Concentration
- \(~{{\varvec{J}}_\mathbf{T}}\) :
-
Fluxes that satisfy tight constraints
- \(~{{\varvec{J}}_\mathbf{R}}\) :
-
Fluxes that satisfy relaxed constraints
- \({{\varvec{u}}_\mathbf{w}}\) :
-
Weight of sum of squared errors
- I:
-
Identity matrix
- \(w_{i}^{{{\text{sc}}}}\) :
-
Time-varying values of the weights for all the metabolites
- \(w_{i}^{{\text{u}}}\) :
-
Weight of upper bound
- \({{W}^{\text{u}}}\) :
-
Maximum allowable value for \(w_{i}^{{{\text{sc}}}}\)
- \(w_{i}^{{\text{l}}}\) :
-
Weight of lower bound
- \({N_{\text{C}}}\) :
-
Number of the objective functions’ candidates
- \({{\varvec{n}}_\mathbf{g}}\) :
-
Estimated noise in the growth rate
- \({N_{{\text{SC}}}}\) :
-
Total number of metabolites
- \({N_{\text{m}}}\) :
-
Number of measured metabolites
- \({V_{i,{\text{max}}}}\) :
-
Maximum rate
- \({K_i}\) :
-
Half saturation concentration
- \(\varepsilon\) :
-
Measurement error
- \({X_k}\) :
-
The biomass value at time \(~k\)
- \({w_{{{\text{c}}_i}}}\) :
-
The weight coefficients of the objective function candidates
References
Schilling CH, Letscher D, Palsson BO (2000) Theory for the systemic definition of metabolic pathways and their use in interpreting metabolic function from a pathway-oriented perspective. J Theor Biol 203(3):229–248
Jaqaman K, Danuser G (2006) Linking data to models: data regression. Nat Rev Mol Cell Biol 7(11):813–819
Orth JD, Thiele I, Palsson BO (2010) What is flux balance analysis?. Nat Biotechnol 28(3):245–248
Foguet C et al (2016) HepatoDyn: a dynamic model of hepatocyte metabolism that integrates C-13 isotopomer data. PLoS Comput Biol 12(4):e1004899
Llaneras F, Sala A, Picó J (2012) Dynamic estimations of metabolic fluxes with constraint-based models and possibility theory. J Process Control 22(10):1946–1955
Varma A, Palsson BO (1995) Parametric sensitivity of stoichiometric flux balance models applied to wild-type Escherichia coli metabolism. Biotechnol Bioeng 45(1):69–79
Mahadevan R, Edwards JS, Doyle FJ (2002) Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys J 83(3):1331–1340
Sanchez CEG, Saez RGT (2014) Comparison and analysis of objective functions in flux balance analysis. Biotechnol Prog 30(5):985–991
Pramanik J, Keasling JD (1997) Stoichiometric model of Escherichia coli metabolism: incorporation of growth-rate dependent biomass composition and mechanistic energy requirements. Biotechnol Bioeng 56(4):398–421
Schuetz R, Kuepfer L, Sauer U (2007) Systematic evaluation of objective functions for predicting intracellular fluxes in Escherichia coli. Mol Syst Biol 3:119
Knorr AL, Jain R, Srivastava R (2007) Bayesian-based selection of metabolic objective functions. Bioinformatics 23(3):351–357
Burgard AP, Maranas CD (2003) Optimization-based framework for inferring and testing hypothesized metabolic objective functions. Biotechnol Bioeng 82(6):670–677
Savinell JM, Palsson BO (1992) Network analysis of intermediary metabolism using linear optimization.1. Development of mathematical formalism. J Theor Biol 154(4):421–454
Gianchandani EP et al (2008) Predicting biological system objectives de novo from internal state measurements. BMC Bioinform 9:43
Llaneras F, Picó J (2008) Stoichiometric modelling of cell metabolism. J Biosci Bioeng 105(1):1–11
Nikdel A, Budman H (2016) Identification of active constraints in dynamic flux balance analysis. Biotechnol Prog 33(1):26–36
Maranas CD, Zomorrodi AR, Wiley Online Library (2016) Optimization methods in metabolic networks, Wiley, Hoboken (1 online resource)
Borchers S et al (2013) Identification of growth phases and influencing factors in cultivations with AGE1.HN cells using set-based methods. PLoS One 8(8):e68124
Shuler ML, Kargi F (1992) Bioprocess engineering: basic concepts. Prentice Hall international series in the physical and chemical engineering sciences, Prentice Hall, Englewood Cliffs, pp 16+479 s
Thalen M et al (2006) Fed-batch cultivation of Bordetella pertussis: metabolism and Pertussis Toxin production. Biologicals 34(4):289–297
Budman H et al (2013) A dynamic metabolic flux balance based model of fed-batch fermentation of Bordetella pertussis. Biotechnol Prog 29(2):520–531
Weiss JN (1997) The Hill equation revisited: uses and misuses. Faseb J 11(11):835–841
Acknowledgements
The authors would like to thank Natural Science and Engineering Research Council (NSERC).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix I

- J1:
-
39.43 Ac + 35O2 \(~ \to ~\) X
- J2:
-
9.46Glc + 12.92O2 \(~ \to ~\) X
- J3:
-
9.84 Glc + 12.73O2 \(~ \to ~\) 1.24Ac + X
- J4:
-
19.23Glc \(~ \to ~\) 12.12Ac + X
Appendix II
- J1:
-
Pyruvate \(~ \to ~\) PEP
- J2:
-
Pyruvate + CoA \(\to ~\) Acetyl-coA + CO2
- J3:
-
Acetyl-CoA + H2O + oxaloacetate \(\to\) citrate + CoA
- J4:
-
Citrate \(\to \alpha\)-ketoglutarate + CO2
- J5:
-
\(\alpha\)-ketoglutarate + enz-N6 \(~ \to\) succinyl transf. + CO2
- J6:
-
Succinyl transf. + CoA \(\to ~\) succinyl-CoA + enz-N6
- J7:
-
Succinyl-CoA + phosphate \(\to\) CoA + succinate
- J8:
-
Succinate + acceptor \(\to\) fumarate + reduction acceptor
- J9:
-
Fumarate + H2O \(~ \to\) malate
- J10:
-
Malate \(~ \to\) oxaloacetate
- J11:
-
Glutamate + NH3 \(~ \to ~\) phosphate + glutamine
- J12:
-
2 Glutamate \(~ \leftarrow ~\) glutamine + \(~\alpha\)-ketoglutarate
- J13:
-
Glutamate + H2O \(~ \to \alpha\)-ketoglutarate + NH3
- J14:
-
Proline + 2H2O \(~ \to\) glutamate
- J15:
-
Oxaloacetate + glutamate \(\to ~\) aspartate + \(\alpha\)-ketoglutarate
- J16:
-
Aspartate + NH3 \(~ \leftarrow ~\) aspargine
- J17:
-
PEP + \({\text{HCO}}_{3}^{ - }~ \to ~\) phosphate + oxaloacetate*
- J18:
-
Lactate \(~ \to ~\) pyruvate
- J19:
-
Acetate + CoA \(~ \leftarrow ~\) acetyl-CoA + phosphate
- J20:
-
2 Acetyl-CoA \(~ \to ~\) CoA + acetoacetyl-CoA
- J21:
-
Acetoacetyl-CoA \(~ \to ~\) PHB
- J22:
-
Glucose-6-phosphate + 3 glyceraldehyde-3-phosphate \(~ \to ~\) 3 ribose-5-phosphate
- J23:
-
Acetyl-CoA + carrier-protein \(\to ~\) acetoacetyl-carrier + CoA + CO2
- J24:
-
Threonine + 3 pyruvate + 2 glutamate + acetyl-CoA + H2O \(\to ~\) NH3 + 3CO2 + 2H2O + isoleucine + \(~\alpha\)-ketoglutarate + valine + CoA + leucine
- J25:
-
Serine + tetrahydrofolate \(\leftarrow \to\) 5,10-methylenetetrahydrofolate + glycine + H2O
- J26:
-
Serine \(~ \leftarrow \to\) pyruvate + NH3
- J27:
-
Threonine \(~ \leftarrow \to ~\) glycine + acetaldehyde
- J28:
-
Aspartate \(\to\) threonine + phosphate
- J29:
-
Hydrogen sulfide + acetyl-CoA + serine \(\to ~\) CoA + cysteine + acetate
- J30:
-
Glutamate + pyruvate \(\leftarrow \alpha\)-ketoglutarate + alanine
- J31:
-
Aspartate + pyruvate + glutamate + succynyl-CoA \(~ \to ~\) phosphate + \(~\alpha\)-ketogultarate + succinate + lysine + CO2 + CoA
- J32:
-
Malate \(~ \to ~\) pyruvate + CO2
- J33:
-
Amino acids \(~ \to ~\) biomass
- J34:
-
Amino acids \(~ \to ~\) pertactin
- J35:
-
PEP \(~ \to\) glyceraldehyde 3-P + phosphate
- J36:
-
2GAP + H2O \(\to\) glucose 6-P + phosphate
- J37:
-
J 3
- J38:
-
Amino acids \(~ \to ~\) Pertussis toxin
- J39:
-
Amino acids \(~ \to ~\) fimbria
- J40:
-
Amino acids \(\to\) FHA
- J41:
-
Inverse of J14
- J42:
-
Inverse of J27†
- J43:
-
Inverse of J30
- J44:
-
2 Glutamate \(+{\text{~}}\) aspartate \(\to\) fumarate \(+{\text{~}}\alpha\)-ketoglutarate \(~+\) arginine
- J45:
-
Glucose \(~+\) 6-P GAP \(+{\text{~}}\) 2 PEP \(~+{\text{~}}\) glutamate \(\to {\text{~}}\)tyrosine \(+\) d-xylose \(+{\text{~}}\) α-ketoglutarate
- J46:
-
Inverse of J26
- J47:
-
Valine \(+{\text{~}}\) α-ketoglutarate \(\to\) glutamate \(~+\) 4 methyl-2-oxopentanoate
- J48:
-
2 pyruvate \(+\) serine \(+\) aspartate \(+\) cysteine \(\to\) CoA \(+\) 3 CO2 \(+\) glycine
- J49:
-
Inverse of J18
Stoichiometry of purines
Adenine: pyruvate \(~+{\text{~}}\) 2 glutamine \(+\) 2 aspartate \(+{\text{~}}\) glycine \(\to\) adenine \(+{\text{~}}\) 2 glutamate \(+{\text{~}}\) fumarate
Guanine: pyruvate \(+\) 3 glutamine \(+\) aspartate \(+\) glycine \(\to {\text{~}}\) guanine \(+\) 3 glutamate \(+\) fumarate
Stoichiometry of pyrimidines
UMP (Uridylic acid): glutamine \(+\) \({\text{HCO}}_{3}^{ - }+\) aspartate \(+\) ribose-5-phosphate \(\to\) UMP \(+\) CO2 \(+\) glutamate
CMP (Cytidylic acid): glutamine \(+{\text{HCO}}_{3}^{ - }\) þ aspartate \(+\) ribose-5-phosphate \(+\) NH3 \(\to\) CMP \(+\) CO2 \(+\) glutamate
TMP (Thymidylic acid): \({\text{HCO}}_{3}^{ - }\) \(+\) serine \(+\) glutamine \(+\) aspartate \(+\) ribose-5-phosphate \(\to\) glycine \(+\) TMP \(+\) glutamate \(+\) CO2
Metabolite | Value |
|---|---|
Ala | − 1.028497997 |
Arg | − 0.023 |
Asp | − 0.209047 |
Asn | − 0.029 |
Gln | 0.6234 |
Glu | − 3.623564 |
Gly | − 0.635784 |
His | − 0.173987138 |
Ileu | − 0.253078 |
Leu | − 0.26459 |
Lys | − 0.232495 |
Meth | − 0.45017 |
Phe | − 0.028945 |
Pro | − 1.532302 |
Ser | − 2.423 |
Thr | − 0.02846 |
Try | 0 |
Tyr | − 0.00823 |
Val | − 0.92 |
Cys | 0 |
Pyr | 0 |
AcCoA | 0 |
GAP | 0 |
Lac | 7.125 |
Ammonia | 6.2675 |
Co2 | 5.243 |
Α-Ketoglutarate | 0 |
Biomass | 200 |
Pertactin | 0.4171 |
Citrate | 0.50043 |
Succ-transferase | 0 |
Succ CoA | 0 |
Succinate | 0 |
Fumarate | 0 |
Malate | 0 |
Oxaloacetate | 0.005 |
Ribose | 8 |
G6P | 0.4184 |
PEP | 0 |
AcAcCoA | 0 |
PHB | 0 |
FFA | 0.234 |
Pt toxin | 0.33 |
Fimbria | 0.31243 |
FHA | 0.334125 |
Xylose | 0 |
CoA | 0 |
ATP | 0 |
Rights and permissions
About this article
Cite this article
Nikdel, A., Braatz, R.D. & Budman, H.M. A systematic approach for finding the objective function and active constraints for dynamic flux balance analysis. Bioprocess Biosyst Eng 41, 641–655 (2018). https://doi.org/10.1007/s00449-018-1899-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1899-y