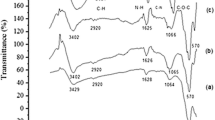
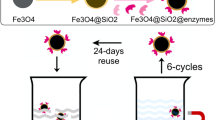
Abstract
Magnetic biocatalysts offer enormous advantages over traditional ones. Their ability to be isolated by means of a magnet, in combination with their extensive reuse possibilities, makes them highly attractive and competitive from the commercial point of view. In this work, magnetic biocatalysts were prepared by immobilization of Candida antarctica Lipase B (E.C. 3.1.1.3, CALB) on magnetite–lysine nanoparticles. Two methodologies were explored tending to find the optimal biocatalyst in terms of its practical implementation: I—physical adsorption of CALB followed by cross-linking, and II—covalent coupling of the lipase on the nanoparticles surface. Both procedures involved the use of glutaraldehyde (GLUT) as cross-linker or coupling agent, respectively. A range of GLUT concentrations was evaluated in method I and the optimum one, in terms of efficiency and operational stability, was chosen to induce the covalent linkage CALB-support in method II. The chosen test reaction was solvent-free ethyl oleate synthesis. Method I produced operationally unstable catalysts that deactivated totally in four to six cycles. On the other hand, covalently attached CALB (method II) preserved 60% of its initial activity after eight cycles and also retained 90% of its initial activity along 6 weeks in storage. CALB immobilization by covalent linkage using controlled GLUT concentration appears as the optimum methodology to asses efficient and stable biocatalysts. The materials prepared within this work may be competitive with commercially available biocatalysts.











Similar content being viewed by others
References
Wu L, Wu S, Xu Z et al (2016) Modified nanoporous titanium dioxide as a novel carrier for enzyme immobilization. Biosens Bioelectron 80:59–66
Yuce-Dursun B, Cigil AB, Dongez D et al (2016) Preparation and characterization of sol–gel hybrid coating films for covalent immobilization of lipase enzyme. J Mol Catal B Enzym 127:18–25
Lv JS, Liu XY, Xu JX et al (2013) Preparation and properties of adsorption material from corn stalks core when used for enzyme immobilization and the subsequent activities of the adsorbed enzymes. Ind Crops Prod 50:787–796
García-García MI, Sola-Carvajal A, Sánchez-Carrón G et al (2011) New stabilized FastPrep-CLEAs for sialic acid synthesis. Bioresour Technol 102:6186–6191
Santos JCS dos, Barbosa O, Ortiz C et al (2015) Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 7:2413–2432
Bezerra RM, Neto DMA, Galvão WS et al (2017) Design of a lipase-nano particle biocatalysts and its use in the kinetic resolution of medicament precursors. Biochem Eng J 125:104–115
Cipolatti EP, Valério A, Henriques RO et al (2016) Nanomaterials for biocatalyst immobilization—state of the art and future trends. RSC Adv 6:104675–104692
Liu X, Chen X, Li Y et al (2012) Preparation of superparamagnetic Fe3O4@alginate/chitosan nanospheres for candida rugosa lipase immobilization and utilization of layer-by-layer assembly to enhance the stability of immobilized lipase. ACS Appl Mater Interfaces 4:5169–5178
Sui Y, Cui Y, Nie Y et al (2012) Surface modification of magnetite nanoparticles using gluconic acid and their application in immobilized lipase. Colloids Surf B Biointerfaces 93:24–28
Swarnalatha V, Aluri Esther R, Dhamodharan R (2013) Immobilization of α-amylase on gum acacia stabilized magnetite nanoparticles, an easily recoverable and reusable support. J Mol Catal B Enzym 96:6–13
Durdureanu-Angheluta A, Ignat ME, Maier SS et al (2014) Lipolytic biocatalyst based on recyclable magnetite-polysiloxane nanoparticles. Appl Surf Sci 292:898–905
Meng X, Xu G, Zhou Q-L et al (2014) Highly efficient solvent-free synthesis of 1,3-diacylglycerols by lipase immobilised on nano-sized magnetite particles. Food Chem 143:319–324
Reis P, Holmberg K, Watzke H et al (2009) Lipases at interfaces: a review. Adv Colloid Interface Sci 147:237–250
Villalba M, Verdasco-Martín CM, dos Santos JCS et al (2016) Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzyme Microb Technol 90:35–44
Manoel EA, Ribeiro MFP, dos Santos JCS et al (2015) Accurel MP 1000 as a support for the immobilization of lipase from Burkholderia cepacia: application to the kinetic resolution of myo-inositol derivatives. Process Biochem 50:1557–1564
Lozano P, De Diego T, Carrie D et al (2003) Lipase catalysis in ionic liquids and supercritical carbon dioxide at 150 °C. Biotechnol Prog 19:380–382
Lozano P, De Diego T, Carrié D et al (2001) Over-stabilization of Candida antarctica lipase B by ionic liquids in ester synthesis. Biotechnol Lett 23:1529–1533
Nicolás P, Lassalle VL, Ferreira ML (2015) About the role of typical spacer/crosslinker on the design of efficient magnetic biocatalysts based on nanosized magnetite. J Mol Catal B Enzym 122:296–304
José C, Bonetto RD, Gambaro LA et al (2011) Investigation of the causes of deactivation–degradation of the commercial biocatalyst Novozym® 435 in ethanol and ethanol–aqueous media. J Mol Catal B Enzym 71:95–107
Nicolás P, Lassalle V, Ferreira ML (2014) Development of a magnetic biocatalyst useful for the synthesis of ethyloleate. Bioprocess Biosyst Eng 37:585–591
Gotor-Fernández V, Busto E, Gotor V (2006) Candida antarctica lipase B: an ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv Synth Catal 348:797–812
Khan NR, Rathod VK (2015) Enzyme catalyzed synthesis of cosmetic esters and its intensification: a review. Process Biochem 50:1793–1806
Derewenda U, Brzozowski AM, Lawson DM, Derewenda ZS (1992) Catalysis at the interface: the anatomy of a conformational change in a triglyceride lipase. BioChemistry 31:1532–1541
Manoel EA, dos Santos JCS, Freire DMG et al (2015) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Technol 71:53–57
Cao L (2006) Carrier-bound immobilized enzymes: principles, application and design, Wiley, Weinheim
Talbert JN, Wang LS, Duncan B et al (2014) Immobilization and stabilization of lipase (CaLB) through hierarchical interfacial assembly. Biomacromolecules 15:3915–3922
Ziegler-Borowska M, Siódmiak T, Chełminiak D et al (2014) Magnetic nanoparticles with surfaces modified with chitosan–poly[N-benzyl-2-(methacryloxy)-N,N-dimethylethanaminium bromide] for lipase immobilization. Appl Surf Sci 288:641–648
Kanimozhi S, Perinbam K (2013) Synthesis of amino-silane modified superparamagnetic Fe3O4 nanoparticles and its application in immobilization of lipase from Pseudomonas fluorescens Lp1. Mater Res Bull 48:1830–1836
Cui Y, Li Y, Yang Y et al (2010) Facile synthesis of amino-silane modified superparamagnetic Fe3O4 nanoparticles and application for lipase immobilization. J Biotechnol 150:171–174
Bezbradica DI, Mateo C, Guisan JM (2014) Novel support for enzyme immobilization prepared by chemical activation with cysteine and glutaraldehyde. J Mol Catal B Enzym 102:218–224
Zhong H, Fang Z, Zou B et al (2013) Studies on the lipase-catalyzed esterification of alkyl oleates in solvent-free systems. J Mol Catal B Enzym 90:114–117
Llerena-Suster CR, Briand LE, Morcelle SR (2014) Analytical characterization and purification of a commercial extract of enzymes: a case study. Colloids Surf B Biointerfaces 121:11–20
Nicolás P, Lassalle VL, Ferreira ML (2016) Quantification of immobilized Candida antarctica lipase B (CALB) using ICP-AES combined with Bradford Method. Enzyme Microb Technol 97:97–103
Azcona P, Zysler R, Lassalle V (2016) Simple and novel strategies to achieve shape and size control of magnetite nanoparticles intended for biomedical applications. Colloids Surf A Physicochem Eng Asp 504:320–330
Foresti ML, Ferreira ML (2005) Frequent analytical/experimental problems in lipase-mediated synthesis in solvent-free systems and how to avoid them. Anal Bioanal Chem 381:1408–1425
Foresti ML, Pedernera M, Bucalá V, Ferreira ML (2007) Multiple effects of water on solvent-free enzymatic esterifications. Enzyme Microb Technol 41:62–70
Metzler DE (2001) Biochemistry: the chemical reactions of living cells. Elsevier, Amsterdam
Nicolás P, Saleta M, Troiani H et al (2013) Preparation of iron oxide nanoparticles stabilized with biomolecules: experimental and mechanistic issues. Acta Biomater 9:4754–4762
Shu G, Chen H, Zhang Q, Dang Y (2013) The effect of glutaraldehyde cross-linking on the enzyme activity of immobilized β-galactosidase on chitosan bead. Adv J Food Sci Technol 5:932–935
Dinçer A, Becerik S, Aydemir T (2012) Immobilization of tyrosinase on chitosan–clay composite beads. Int J Biol Macromol 50:815–820
Liu K, Zhao G, He B et al (2012) Immobilization of pectinase and lipase on macroporous resin coated with chitosan for treatment of whitewater from papermaking. Bioresour Technol 123:616–619
Barbosa O, Torres R, Ortiz C, Fernandez-Lafuente R (2012) Versatility of glutaraldehyde to immobilize lipases: effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem 47:1220–1227
Monsan P (1978) Optimization of glutaraldehyde activation of a support for enzyme immobilization. J Mol Catal 3:371–384
López-Gallego F, Betancor L, Mateo C et al (2005) Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J Biotechnol 119:70–75
Hua L, Sun Z-H, Zheng P, Xu Y (2004) Biocatalytic resolution of dl-pantolactone by glutaraldehyde cross-linked cells of Fusarium moniliforme CGMCC 0536. Enzyme Microb Technol 35:161–166
Zhang W-W, Yang X-L, Jia J-Q et al (2015) Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J Mol Catal B Enzym 115:83–89
Guauque Torres MP, Foresti ML, Ferreira ML (2013) Effect of different parameters on the hydrolytic activity of cross-linked enzyme aggregates (CLEAs) of lipase from Thermomyces lanuginosa. Biochem Eng J 72:18–23
Guauque Torres MP, Foresti ML, Ferreira ML (2014) CLEAs of Candida antarctica lipase B (CALB) with a bovine serum albumin (BSA) cofeeder core: Study of their catalytic activity. Biochem Eng J 90:36–43
Lage FAP, Bassi JJ, Corradini MCC et al (2016) Preparation of a biocatalyst via physical adsorption of lipase from Thermomyces lanuginosus on hydrophobic support to catalyze biolubricant synthesis by esterification reaction in a solvent-free system. Enzyme Microb Technol 84:56–67
Walt DR, Agayn VI (1994) The chemistry of enzyme and protein immobilization with glutaraldehyde. TrAC Trends Anal Chem 13:425–430
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–802
Velu N, Divakar K, Nandhinidevi G, Gautam P (2012) Lipase from Aeromonas caviae AU04: isolation, purification and protein aggregation. Biocatal Agric Biotechnol 1:45–50
Betancor L, López-Gallego F, Hidalgo A et al (2006) Different mechanisms of protein immobilization on glutaraldehyde activated supports: effect of support activation and immobilization conditions. Enzyme Microb Technol 39:877–882
Fernández-Lorente G, Palomo JM, Mateo C et al (2006) Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules 7:2610–2615
Barbosa O, Ortiz C, Berenguer-Murcia Á et al (2015) Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol Adv 33:435–456
Gunda NSK, Singh M, Norman L et al (2014) Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl Surf Sci 305:522–530
Silva JA, Macedo GP, Rodrigues DS et al (2012) Immobilization of Candida antarctica lipase B by covalent attachment on chitosan-based hydrogels using different support activation strategies. Biochem Eng J 60:16–24
dos Santos P, Rezende CA, Martinez J (2016) Activity of immobilized lipase from Candida antarctica (lipozyme 435) and its performance on the esterification of oleic acid in supercritical carbon dioxide. J Supercrit Fluids 107:170–178
Jiang Y, Zheng P, Zhou L et al (2016) Immobilization of lipase in hierarchically ordered macroporous/mesoporous silica with improved catalytic performance. J Mol Catal B Enzym 130:96–103
Ajmal M, Fieg G, Keil F (2016) Analysis of process intensification in enzyme catalyzed reactions using ultrasound. Chem Eng Process Process Intensif 110:106–113
dos Santos JCS, Bonazza HL, de Matos LJBL et al (2017) Immobilization of CALB on activated chitosan: application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnol Reports 14:16–26
José C, Austic GB, Bonetto RD et al (2013) Investigation of the stability of Novozym® 435 in the production of biodiesel. Catal Today 213:73–80
Foresti ML, Ferreira ML (2007) Chitosan-immobilized lipases for the catalysis of fatty acid esterifications. Enzyme Microb Technol 40:769–777
Sun J, Jiang Y, Zhou L, Gao J (2010) Immobilization of Candida antarctica lipase B by adsorption in organic medium. N Biotechnol 27:53–58
Toledo MV, José C, Collins SE et al (2012) Esterification of R/S-ketoprofen with 2-propanol as reactant and solvent catalyzed by Novozym® 435 at selected conditions. J Mol Catal B Enzym 83:108–119
Séverac E, Galy O, Turon F et al (2011) Selection of CalB immobilization method to be used in continuous oil transesterification: analysis of the economical impact. Enzyme Microb Technol 48:61–70
Ngo TPN, Li A, Tiew KW, Li Z (2013) Efficient transformation of grease to biodiesel using highly active and easily recyclable magnetic nanobiocatalyst aggregates. Bioresour Technol 145:233–239
Mehrasbi MR, Mohammadi J, Peyda M, Mohammadi M (2017) Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew Energy 101:593–602
Babaki M, Yousefi M, Habibi Z et al (2015) Preparation of highly reusable biocatalysts by immobilization of lipases on epoxy-functionalized silica for production of biodiesel from canola oil. Biochem Eng J 101:23–31
Fedosov SN, Brask J, Pedersen AK et al (2013) Kinetic model of biodiesel production using immobilized lipase Candida antarctica lipase B. J Mol Catal B Enzym 85–86:156–168
Ortega-Requena S, Bódalo-Santoyo A, Bastida-Rodríguez J et al (2014) Optimized enzymatic synthesis of the food additive polyglycerol polyricinoleate (PGPR) using Novozym® 435 in a solvent free system. Biochem Eng J 84:91–97
Ćorović M, Milivojević A, Carević M et al (2017) Batch and semicontinuous production of l-ascorbyl oleate catalyzed by CALB immobilized onto Purolite® MN102. Chem Eng Res Des 126:161–171
Ravelo M, Fuente E, Blanco Á et al (2015) Esterification of glycerol and ibuprofen in solventless media catalyzed by free CALB: kinetic modelling. Biochem Eng J 101:228–236
Acknowledgements
The authors acknowledge the financial support from CONICET (Argentina) and the economic support from PICT 2010-0788 (ANPCyT, Argentina), and the PGI N°24/ZQ09 (UNS, Argentina).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nicolás, P., Lassalle, V. & Ferreira, M.L. Immobilization of CALB on lysine-modified magnetic nanoparticles: influence of the immobilization protocol. Bioprocess Biosyst Eng 41, 171–184 (2018). https://doi.org/10.1007/s00449-017-1855-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1855-2