Abstract
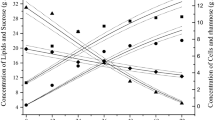
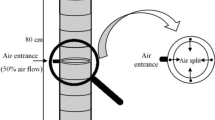
In the present study, extensive experimental investigations and detailed theoretical analysis of a two-dimensional packed bed bioreactor, employed for the production of galacto-oligosaccharides (GOS) from milk whey were performed. Model equations, in one- and two-dimensions, capable of predicting the substrate concentration distribution in the bioreactor were developed by coupling mass balance equation with appropriate velocity distribution equation and solved numerically. Validation of the proposed model equations was done by a set of experimental data obtained from the bioreactor. The effects of reactor to catalyst particle diameter ratio (d t/d p), feed flowrate (10−6–10−9 m3 s−1), and initial lactose concentration (50–200 kg m−3) on substrate concentration distribution were investigated in detail. While, the distribution of substrate concentration in axial direction was independent of d t/d p, it was observed that for d t/d p <40, significant radial concentration distribution existed. It was further observed that the substrate conversion and product yield obtained experimentally showed an excellent agreement (97 ± 2 %) with the results predicted by the two-dimensional model equation, whereas, the results predicted by the one-dimensional model equation did not lie within the desired confidence level (<90 %). The results were confirmed by both curve fitting and statistical analysis. The prediction of substrate concentration distribution in axial and radial directions using the developed two-dimensional model equation is necessary for computing the bioreactor volume to achieve the desired GOS yield.










Similar content being viewed by others
Abbreviations
- A :
-
Area normal to the direction of flow (m2)
- A 1 :
-
First constant in velocity distribution equation
- A 2 :
-
Second constant in velocity distribution equation
- A 3 :
-
Third constant in velocity distribution equation
- B :
-
Fourth constant in velocity distribution equation
- C A :
-
Concentration of lactose (kg m−3)
- C A0 :
-
Inlet lactose concentration (kg m−3)
- CE0 :
-
Concentration of enzyme (kg m−3)
- d p :
-
Diameter of catalyst pellet (m)
- d t :
-
Diameter of the fixed bed bioreactor (m)
- (D e)L :
-
Effective axial diffusivity (m2 s−1)
- (D e)R :
-
Effective radial diffusivity (m2 s−1)
- kcat :
-
Specific reaction rate constant (s−1)
- K m :
-
Michaelis–Menten constant (kg m−3)
- N A :
-
Mass flux (kg m−2 s−1)
- (Pe)L :
-
Axial Peclet number
- (Pe)R :
-
Radial Peclet number
- r :
-
Radial distance (m)
- R :
-
Radius of fixed bed bioreactor (m)
- \( r^{*} \) :
-
Dimensionless radial distance
- r A :
-
Rate of depletion of substrate lactose (kg m−3 s−1)
- u(r):
-
Axial velocity as a function of radial distance (m s−1)
- \( u(r)^{*} \) :
-
Dimensionless radial velocity
- u c :
-
Superficial velocity (m s−1)
- x A :
-
Fractional conversion of lactose
- x i, exp :
-
Conversion (experimental) at a particular axial position i
- x i, simu :
-
Conversion (simulated) at a particular axial position i
- \( x_{i}^{k} \) :
-
Conversion (simulated) after kth iteration
- \( x_{i}^{k + 1} \) :
-
Conversion (simulated) after (k + 1)th iteration
- F (\( x_{i}^{k} \)):
-
Nonlinear function of lactose conversion at a particular axial position i for any radial position
- z :
-
Axial distance (m)
References
Hsu CA, Lee SL, Chou CH (2007) Enzymatic production of galactooligosaccharides by β-galactosidase from bifidobacterium longum BCRC 15708. J Agric Food Chem 55:2225–2230
Kosseva MR (2013) Use of immobilized biocatalyst for valorization of whey lactose. In: Kosseva MR, Webb C (eds) Food industry wastes assessment and recuperation of commodities. Academic Press, San Diego
Gosling A, Stevens GW, Barber AR, Kentish SE, Gras SL (2010) Recent advances refining galactooligosaccharide production from lactose. Food Chem 121:307–318
Rodriguez-Colinas B, Fernandez-Arrojo L, Ballesteros AO, Plou FJ (2014) Galactooligosaccharides formation during enzymatic hydrolysis of lactose: towards a prebiotic-enriched milk. Food Chem 145:388–394
Sen D, Sarkar A, Das S, Chowdhury R, Bhattacharjee C (2012) Batch hydrolysis and rotating disc membrane bioreactor for the production of galacto-oligosaccharides: a comparative study. Ind Eng Chem Res 51:10671–10681
Lopez-Leiva MH, Guzman M (1995) Formation of oligosaccharides during enzymatic hydrolysis of milk whey permeates. Process Biochem 30:757–762
Palai T, Mitra S, Bhattacharya PK (2012) Kinetics and design relation for enzymatic conversion of lactose into galacto-oligosaccharides using commercial grade β-galactosidase. J Biosci Bioeng 114:418–423
Boon MA, Janssen AEM, van’t Riet K (1999) Modelling and parameter estimation of the enzymatic synthesis of oligosaccharides by β-galactosidase from Bacillus circulans. Biotechnol Bioeng 64:558–567
Boon MA, Janssen AEM, van’t Riet K (2000) Effect of temperature and enzyme origin on the enzymatic synthesis of oligo-saccharides. Enzyme Microb Technol 26:271–281
Iwasaki K, Nakajima M, Nakao S (1994) Galacto-oligosaccharide production from lactose by an enzymatic batch reaction using β-galactosidase. Process Biochem 31:69–76
Gosling A, Stevens G, Barber A, Kentish S, Gras S (2011) Effect of the substrate concentration and water activity on the yield and rate of the transfer reaction of β-galactosidase from Bacillus circulans. J Agric Food Chem 59:3366–3372
Jorgensen F, Hansen OC, Stougaard P (2001) High-efficiency synthesis of oligosaccharides with a truncated β-galactosidase from Bifidobacteriumbifidum. Appl Microbiol Biotechnol 57:647–652
Rodriguez-Collinas B, Poveda A, Jimenez-Barbero J, Ballesteros AO, Plou FJ (2012) Galacto-oligosaccharide synthesis from lactose solution or skim milk using the β-galactosidase from Bacillus circulans. J Agric Food Chem 60:6391–6398
Sen P, Nath A, Bhattacharjee C, Chowdhury R, Bhattacharya P (2014) Process engineering studies of free and micro-encapsulated β-galactosidase in batch and packed bed bioreactors for production of galactooligosaccharides. Biochem Eng J 90:59–72
Neri D, Balcão VM, Costa RS, Isabel RCAP, Ferreira EMFC, Torres DPM, Rodrigues LRM, Carvalho LB Jr, Teixeir JA (2009) Galacto-oligosaccharides production during lactose hydrolysis by free Aspergillus oryzae β-galactosidase and immobilized on magnetic polysiloxane-polyvinyl alcohol. Food Chem 115:92–99
Albayrak N, Yang S (2002) Immobilization of Aspergillus oryzae β-galactosidase on tosylated cotton cloth. Enzyme Microb Technol 31:371–383
Albayrak N, Yang S (2002) Production of galacto-oligosaccharides from lactose by Aspergillus oryzae β-galactosidase immobilized on cotton cloth. Biotechnol Bioeng 77:8–19
Nichele V, Signoretto M, Ghedini E (2011) β-Galactosidase entrapment in silica gel matrices for a more effective treatment of lactose intolerance. J Mol Catal B Enzyme 71:10–15
Freitas FF, Marquez LDS, Ribeiro GP, Brandao GC, Cardoso VL, Ribeiro EJ (2011) A comparison of the kinetic properties of free and immobilized Aspergillus oryzae β-galactosidase. Biochem Eng J 58–59:33–38
Vieira DC, Lima LN, Mendes AA, Adriano WS, Giordano RC, Giordano RLC, Tardioli PW (2013) Hydrolysis of lactose in whole milk catalyzed by β-galactosidase from Kluyveromyces fragilis immobilized on chitosan-based matrix. Biochem Eng J 81:54–64
Huerta LM, Vera C, Guerrero C, Wilson L, Illanes A (2011) Synthesis of galacto-oligosachharides at very high lactose concentrations with immobilized β-galactosidases from Aspergillus oryzae. Process Biochem 46:245–252
Neri D, Balcão VM, Cardoso SM, Silva AMS, Domingues MRM, Torres DPM, Rodrigues LRM, Carvalho LB Jr, Teixeira JAC (2011) Characterization of galactooligosachharides produced by β-galactosidase immobilized onto magnetized Dacron. Int Dairy J 21:172–178
Gaur R, Pant H, Jain R, Khare SK (2006) Galacto-oligosaccharide synthesis by immobilized Aspergillus oryzae β-galactosidase. Food Chem 97:426–430
Lu L, Xu S, Zhao R, Zhang D, Li Z, Li Y, Xiao M (2012) Synthesis of galactooligosaccharides by CBD fusion β-galactosidase immobilized on cellulose. Bioresour Technol 116:327–333
Haider T, Husain Q (2009) Hydrolysis of milk/whey lactose by β-galactosidase: a comparative study of stirred batch process and packed bed reactor prepared with calcium alginate entrapped enzyme. Chem Eng Process Process Intensif 48:576–580
Ansari SA, Husain Q (2012) Lactose hydrolysis from milk/whey in batch and continuous processes by concanavalin A-Celite 545 immobilized Aspergillus oryzae β-galactosidase. Food Bioprod Process 90:351–359
Klein MP, Fallavena LP, da Schöffer JN, Ayub MAZ, Rodrigues RC, Ninow JL, Hertz PF (2013) High stability of immobilized β-galactosidase for lactose hydrolysis and galactooligosaccharides synthesis. Carbohydr Polym 95:465–470
Nakkharat P, Haltrich D (2007) β-Galactosidase from Talaromyces thermophilus immobilized on to Eupergit C for production of galacto-oligosaccharides during lactose hydrolysis in batch and packed-bed reactor. World J Microbiol Biotechnol 23:759–764
Sheu DC, Li SY, Duan KJ, Chen CW (1998) Production of galactooligosaccharides by β-galactosidase immobilized on glutaraldehyde-treated chitosan beads. Biotechnol Techn 12:273–276
Akpan E, Akande A, Aboudheir A, Ibrahim H, Idem R (2007) Experimental, kinetic and 2-D reactor modeling for simulation of the production of hydrogen by the catalytic reforming of concentrated crude ethanol (CRCCE) over a Ni-based commercial catalyst in a packed-bed tubular reactor. Chem Eng Sci 62:3112–3126
Merrill LS Jr, Hamrin CE Jr (1970) Conversion and temperature profiles for complex reactions in laminar and plug flow. AIChE J 16:194–198
Mousazadeh F, van Den Akker HEA, Mudde RF (2013) Direct numerical simulation of an exothermic gas-phase reaction in a packed bed with random particle distribution. Chem Eng Sci 100:259–265
Masmoudi MA, Sahraoui M, Grioui N, Halouani K (2014) 2-D modeling of thermo-kinetics coupled with heat and mass transfer in the reduction zone of a fixed bed downdraft biomass gasifier. Renew Energy 66:288–298
Lerou JJ, Froment GF (1977) Velocity, temperature and conversion profiles in fixed bed catalytic reactors. Chem Eng Sci 32:853–861
Krause P, Fieg G (2011) Experiment based model development for enzymatic hydrolysis in a packed-bed reactor with biphasic reactant flow. Chem Eng Sci 66:4838–4850
Delmas H, Froment GF (1988) A simulation model accounting for structural radial nonuniformities in fixed bed reactors. Chem Eng Sci 43:2281–2287
Smith JM (1970) Chemical engineering kinetics. McGraw-Hill, New York
Blanch HW, Clark DS (1996) Biochemical engineering. CRC Press, New York
Schwartz CE, Smith JM (1953) Flow distribution in packed beds. Ind Eng Chem Res 45:1209–1218
Kufner R, Hofman H (1990) Implementation of radial porosity and velocity distribution in a reactor model for heterogenous catalytic gasphase reactions (Torus-Model). Chem Eng Sci 45:2141–2146
Ahmed M, Fahien RW (1980) Tubular reactor design-I two dimensional model. Chem Eng Sci 35:889–895
Godini HR, Xiao S, Kim M, Holst N, Jaso S, Gorke O, Steinbach J, Wozny G (2014) Experimental and model-based analysis of membrane reactor performance for methane oxidative coupling: effect of radial heat and mass transfer. J Ind Eng Chem 20:1993–2002
Constantinides A, Mostoufi N (1999) Numerical methods for chemical engineers with MATLAB applications. NJ Prentice Hall PTR, New Jersey
Gupta SK (1995) Numerical methods for engineers. New Age, New Delhi
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Mateo C, Monti R, Pessela BCC, Fuentes M, Torres R, Guisan JM, Fernandez-Lafuente R (2004) Immobilization of lactose from Kluyveromyces lactis greatly reduces the inhibition promoted by glucose. Full hydrolysis of lactose in milk. Biotechnol Prog 20:1259–1262
Jovanovic-Malinovska R, Fernandes P, Winkelhausen E, Fonseca L (2012) Galacto-oligosaccharides synthesis from lactose and whey by β-galactosidase immobilized in PVA. Appl Biochem Biotechnol 168:1197–1211
Feng Y, Chang X, Wang W, Ma R (2000) Artificial cells. Stabilities of immobilized β-galactosidase of Aspergillus sp. AF for the optimal production of galactooligosaccharides from lactose. Blood Subs Biotechnol 38:43–51
Shin HJ, Park JM, Yang JW (1998) Continuous production of galactooligosaccharides from lactose by Bullera singularis β-galactosidase immobilized in chitosan beads. Process Biochem 33:787–792
Vera C, Guerrero C, Conejeros R, Illanes A (2012) Synthesis of galacto-oligosaccharides by β-galactosidase from Aspergillus oryzae using partially dissolved and supersaturated solution of lactose. Enzyme Microb Technol 50:188–194
Dwevedi A, Kayastha AM (2009) Stabilization of β-galactosidase (from Peas) by immobilization onto amberlite MB-150 beads and its application in lactose hydrolysis. J Agric Food Chem 57:682–688
Selvarajan E, Mohanasrinivasan V, Devi CS, Doss CGP (2015) Immobilization of β-galactosidase from Lactobacillus plantarum HF571129 on ZnO nanoparticles: characterization and lactose hydrolysis. Bioprocess Biosyst Eng. doi:10.1007/s00449-0.15-1407-6
Acknowledgments
The authors are grateful to the University Grants Commission (UGC) UPE Phase II for their financial support to carry out the present work.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The governing differential Eq. (15a) along with the boundary conditions given by Eqs. (16)–(19) was used for predicting concentration distribution in the axial and the radial directions. The equation was solved by finite difference method through the method of lines outlined as follows:
One chooses N + 1 equispaced finite-difference grid lines, \( r_{1}^{*} ( = 0), \, r_{2}^{*} , \, r_{3}^{*} , \ldots ,r_{N + 1}^{*} ( = R/d_{\text{p}} ) \) with the spacing being \( \Delta r^{*} = (R/d_{\text{p}} )/N \). Here N = 6 and the value of x A at the ith grid line are denoted by \( x_{A} \left( {r_{i}^{*} ,z} \right) = x_{Ai} \left( z \right) \); \( i = 1,2, \ldots N + 1 \). The partial derivatives in r may be written as:
It is implied here that the value of z used for all the x A,i ’s is the same. The residuals can now be equated to zero at the appropriate grid lines for Eq. (15a).
This leads to:
where, \( i = 1,2, \ldots N + 1. \)
Here, the velocity profile \( u(r_{i}^{*} ) \) is given by Eq. (10).
At \( r^{*} = r_{1}^{*} \), the boundary condition on x A gives:
where, \( r^{*} = r_{0}^{*} \) is a hypothetical grid line, at which conversion is x A (z). x A (z) can be eliminated by making the residual of the PDE at \( r^{*} = r_{1}^{*} \) equal to zero, which leads to:
The term \( \frac{1}{{r^{*} }}\frac{{\partial x_{A} }}{{\partial r^{*} }} \) gives 0/0 at r = r 1 = 0. L′Hospital’s rule applied to this term reduces it to \( \frac{{\partial^{2} x_{A} }}{{\partial r^{*2} }} \). Thus, the terms in bracket on the right hand side become \( 2\frac{{\partial^{2} x_{A} }}{{\partial r^{*2} }} \). Using the finite difference form, one gets:
which upon rearranging becomes:
For \( i = 2, \ldots N, \) Eq. (27) reduces to:
At \( r^{*} = r_{N + 1}^{*} = R/d_{\text{p}} \), Eq. (27) becomes:
Because of the no slip boundary condition at the wall, (r N+1 = R), a region very close to the wall has been considered to be r N+1,where the velocity is very small but has a non-zero value.
The set of PDEs represented by Eq. (28) has thus reduced to a set of second order ordinary differential equations under the (ODE-BVPs) in z direction as given by Eqs. (31)–(33). One chooses M + 1 equispaced finite-difference grid points \( z_{1}^{*} ( = 0), \, z_{2}^{*} , \, z_{3}^{*} , \ldots ,z_{M + 1}^{*} ( = L/d_{\text{p}} ) \) with the spacing being \( \Delta z^{*} = \left( {L/d_{\text{p}} } \right)/M \), where M = 19.
For \( r^{*} = r_{1}^{*} \), Eq. (31) may be expressed as:
At \( z^{*} = 0 \), the boundary condition represented by Eq. (16) reduces to:
Here, in x A,11, the first subscript 1 signifies the radial position \( r_{1}^{*} \) and the second subscript 1 signifies the axial position \( z_{1}^{*} \), corresponding to \( r_{1}^{*} \).
Hence, substituting the boundary condition (35) in equation (34) and rearranging, one gets:
which may be written as:
For \( z_{j}^{*} = z_{2}^{*} , \ldots z_{M}^{*} \) Eq. 34 may be written as:
At \( z_{i}^{*} = z_{M + 1}^{*} ( = L/d_{\text{p}} ), \) the boundary condition given by Eq. (17) reduces to:
which when substituted in equation (38) yields:
Equations identical to (37) can be written for equation (38) and (40) also. The equations can be rearranged in matrix form and written as:
Here, \( A\left[ {x_{A,ij}^{k} } \right] \) is the Jacobian matrix \( \frac{\partial F}{\partial x} \)
Similarly, the matrix can be constructed for \( i = 2, \ldots N \) and \( j = 1, \ldots M + 1 \) on rearranging Eq. (27), and solved to obtain the substrate conversion in radial and axial directions.
Rights and permissions
About this article
Cite this article
Sen, P., Bhattacharjee, C. & Bhattacharya, P. Experimental studies and two-dimensional modelling of a packed bed bioreactor used for production of galacto-oligosaccharides (GOS) from milk whey. Bioprocess Biosyst Eng 39, 361–380 (2016). https://doi.org/10.1007/s00449-015-1516-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1516-2