Abstract
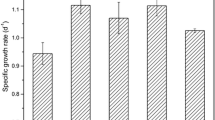
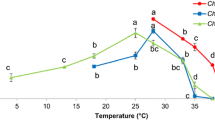
Tunisian microalgae are diverse and rarely been studied. This study reports a first investigation of thermophile Chlorophyta isolated from mats community colonizing the geothermal springs in the north of Tunisia at water temperature 60 °C. In the study, the combined effect of temperature and light intensity was investigated on the cell growth, the mother and daughter cells abundance and the extracellular polymeric substances synthesis in batch culture of the isolated species. Three levels were tested for each factor, 20, 30, 40 °C for temperature; and 20, 70, 120 μmol photons m−2 s−1 for light intensity, using full factorial design and response surface methodology. The thermophile strain was identified as a genus Graesiella and showed 99.8 % similarity with two Graesiella species: Graesiella emersonii and Graesiella vacuolata based on the 18S rDNA molecular identification. The optimal growth condition was found at 30 °C and 120 µmol photons m−2 s−1 (7 MC mL−1 day−1), with the abundance of vegetative cells (daughter cells). In contrast, the number of mother cells increased significantly as the growth decreased; consequently, the highest ratio of auto spore mother cells versus daughter cells (19.4) was obtained at 20 °C and 20 µmol photons m−2 s−1. The highest yield of EPS production (11.7 mg L−1 day−1) was recorded at the highest temperature (40 °C) and lowest light intensity (20 µmol photons m−2s−1). These results revealed how the species respond to high and low temperatures and suggest that the species should be considered as facultative thermophile.




Similar content being viewed by others
References
Zakaria AM, Abdulrahman MA (2007) Cyanobacteria and their toxins in treated-water storage reservoirs in Abha city, Saudi Arabia. Toxicon 50:75–84
Wolfstein K, Stal LJ (2002) Production of extracellular polymeric substances (EPS) by benthic diatoms: effect of irradiance and temperature. Mar Ecol Prog Ser 236:13–22
McKew JD, Taylor TJ, Mcgenity, Underwood GJC (2011) Resilience of benthic biofilm communities from a temperate salt marsh to desiccation and rewetting. ISME J 5:30–41
Thornton DCO (2009) Effect of low pH on carbohydrate production by a marine planktonic diatom (Chaetoceros muelleri). Res. Lett. in Ecol. 2009, Article ID 105901 doi:10.1155/2009/105901
Vu B, Chen M, Russell JC, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14:2535–2554
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, Chermak D, Antony G, White FF, Somerville SC, Mudgett MB, Frommer WB (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532
Guzmán S, Gato A, Lamela M, Freire-Garabal M, Calleja JM (2003) Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res 17:665–670
Zheng W, Chen C, Cheng Q, Wang Y, Chu C (2006) Oral administration of exopolysaccharide from Aphanothece halophytica (Chroococcales) significantly inhibits influenza virus (H1N1)-induced pneumonia in mice. Int Immunopharmacol 6:1093–1099
Dvir I, Chayoth R, Sod-Moriah U, Shany S, Nyska A, Stark AH (2000) Soluble polysaccharide and biomass of red micro algae Porphyridium sp. alter intestinal morphology and reduce serum cholesterol in rats. Br J Nutr 84:469–476
Gardeva E, Toshkova R, Minkova K, Gigova L (2009) Cancer protective action of polysaccharide derived from micro algae Porphyridium cruentum—a biological background. Biotechnol Equip 23:783–787
Seckbach J (2007) Algae and Cyanobacteria in extreme environments. Springer, Dordrecht
Jonker CZ, Van Ginkel C, Olivier J (2013) Association between physical and geochemical characteristics of thermal springs and algal diversity in Limpopo Province, South Africa. Water S.A 39: 95−104
Ratha SK, Prasanna R (2012) Bioprospecting microalgae as potential sources of ‘green energy’, challenges and perspectives. Appl Biochem Microbiol 48:109–125
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of high added-value compounds—a brief review of recent work. Biotechnol Prog 27:597–613
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing micro algal strains for sustainable biofuel production. Bioresource Technol 102:57–70
Radchenkova N, Tomova A, Kambourova M (2011) Biosynthesis of an exopolysaccharide produced by Brevibacillus thermoruber 438. Biotechnol Biotechnol Equip 25:77–79
Carvalho AP, Malcata FX (2003) Kinetic modeling of the autotrophic growth of Pavlova lutheri: study of the combined influence of light and temperature. Biotechnol Prog 19:1128–1135
Trabelsi L, Ben Ouada H, Bacha H, Ghoul M (2009) Combined effect of temperature and light intensity on growth and extracellular polymeric substance production by the Cyanobacterium Arthrospira platensis. Appl Phycol 21:405–412
Guillard RRL (2005) Purification methods for microalgae. In: Andersen RA (ed) Algae culturing techniques. Academic Press, Elsevier Science, San Diego
Bischoff HW, Bold HC (1963) Phycological studies IV. some soil algae from enchanted rock and related algal species. University of Texas Publication 6318:1–95
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LJ (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Lefranc MP, Clément O, Kaas Q, Duprat E, Chastellan P, Coelho I, Combres K, Ginestoux C, Giudicelli V, Chaume D, Lefranc G (2005) IMGT-Choreography for immunogenetics and immunoinformatics. In Silico Biology 5:45–60
Medlin L, Elwood HJ, Stickel S, Sogin ML (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491–499
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res 25:3389–3402
Edgar RC (2004) Local homology recognition and distance measures in linear time using compressed amino acid alphabets. Nucl. Acids Res 32:380–385
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Abbas AR, Wolslegel K, Seshasayee D, Modrusan Z, Clark HF (2009) Deconvolution of blood microarray data identifies cellular activation patterns in systemic Lupus Erythematosus. PLoS one 4:1–16
Nozaki H, Katagiri M, Nakagawa M, Aizawa K, Watanabe MM (1995) Taxonomic re-examination of two strains labeled ‘Chlorella’ in the microbial culture collection at the National Institute for Environmental Studies (NIES-Collection). Microbial Culture Collections 11:11–18
Shihira I, Krauss RW (1965) Chlorella. Physiology and taxonomy of forty-one isolates. University of Maryland, College Park, Maryland, pp 1–97
Rindi F, Lam DW, López-Bautista JM (2009) Phylogenetic relationships and species circumscription in Trentepohlia and Printzina (Trentepohliales, Chlorophyta). Mol Phylogenet Evol 52:329–339
Zhang JM, Huss VAR, Sun XP, Chang KJ, Pang DB (2008) Morphology and phylogenetic position of a trebouxiophycean green alga (Chlorophyta) growing on the rubber tree, Hevea brasiliensis, with the description of a new genus and species. Eur J Phycol 43:185–193
Smith-Bädorf HD, Chuck CJ, Mokebo KR, MacDonald H, Davidson MG, Scott RJ (2013) Bioprospecting the thermal waters of the Roman baths : isolation of oleaginous species and analysis of the FAME profile for biodiesel production. AMB Express 3:9–14
Jackson JEJ, Castenholz RW (1975) Fidelity of thermophilic blue-green algae to hot spring habitats. Limnol Oceanogr 20:305–322
Walsh MM, Seckbach J (1999) The versatility of microorganisms. In: Seckbach J (ed) Enigmatic microorganisms and life in extreme environments. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 153–162
Sorokin C, Krauss RW (1962) Effects of temperature & illuminance on Chlorella growth uncoupled from cell division plant physiol 37:37–42
AVonshak G Torzillo (2004) Environmental stress physiology. In: Richmond A (ed) Microalgal culture: biotechnology and applied phycology. Blackwell Science, pp 5782
Agrawal SC, Manisha (2007) Growth, survival and reproduction in Chlorella vulgaris and C.variegata with respect to culture age and under different chemical factors. Folia Microbiol 52:399–406
Guckert JB, Cooksey KE (1990) A Triglyceride accumulation and fatty aid profile changes in Chlorella (Chlorophyta) during high pH- induced cell cycle inhibition. J Phycol 26:72–79
Rioboo C, O’Connor JE, Prado R, Herrero C, Cid A (2009) Cell proliferation alterations in Chlorella cells under stress conditions. Aquat Toxicol 94:229–237
Linton JD (1991) Metabolite production and growth efficiency. Antonie Van Leeuwenhoek 60:293–311
Otero A, Vincenzini M (2003) Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J Biotechnol 102:143–152
Noffke N, Gerdes G, Klenke T (2003) Benthic cyanobacteria and their influence on the sedimentary dynamics of peritidal depositional systems (siliciclastic, evaporitic salty, and evaporitic carbonatic). Earth-Sciences Reviews 62:163–176
Acknowledgments
The authors would like to give special thanks to Prof. Sami Sayadi (Head of Laboratory of Environmental Bioprocesses) and Dr. Fatma Karray from the Center of Biotechnology of Sfax of Tunisia for their helpful assistance and their collaboration. Also, the authors express their gratitude to Dr. Jun Zhao (Post-doctoral researcher) and Mrs. Haifa Ben Romdhane (Research assistant) at Masdar Institute of Science and Technology, Abu Dhabi, UAE for their critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
449_2014_1204_MOESM1_ESM.tif
Fig. S1 Phylogenetic tree based on the sequence of 18S rDNA (Bootstrap values are given at the nodes, Scale bar represents the substitution percentage, GenBank accession numbers follow species name in parenthesis) (TIFF 11,137 kb)
Rights and permissions
About this article
Cite this article
Mezhoud, N., Zili, F., Bouzidi, N. et al. The effects of temperature and light intensity on growth, reproduction and EPS synthesis of a thermophilic strain related to the genus Graesiella . Bioprocess Biosyst Eng 37, 2271–2280 (2014). https://doi.org/10.1007/s00449-014-1204-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1204-7