Abstract
Migratory bird populations are declining globally at alarming rates. Non-breeding site conditions affect breeding populations, but generalising non-breeding habitat conditions over large spatial regions cannot address potential fine-scale differences across landscapes or local populations. Plumage characteristics can mediate the effects of environmental conditions on individual fitness. However, whether different phenotypes use distinctive non-breeding sites, and whether they respond to non-breeding site conditions differently remains largely unknown. Stable isotopes (δ13C, δ15N, δ2H) of inert tissues are useful to infer habitat characteristics and geographic origins where those tissues were grown. We collected winter-grown feathers from pied flycatchers (Ficedula hypoleuca) on their breeding grounds over several years from males whose dorsal plumage colouration ranged continuously from brown to black and assessed their stable isotope values as proxies of local habitat conditions. Based on feather δ2H profiles we found that browner males spent their non-breeding season in drier habitats than black males. Assignment to origin analysis shows potential regional non-breeding ground separation between differently coloured males. High within-individual repeatability of both δ13C and δ15N indicate the pied flycatcher males return yearly to similar areas. Blacker males were more likely to return to the breeding grounds after dry years compared with brown males. The opposite was found in wet years. Our study demonstrates that different phenotypes are exposed to different non-breeding site conditions which can differentially affect individual survivorship. This has important ramifications for population dynamics under predicted climate change scenarios where especially brown phenotype pied flycatcher males may be under a risk of decreasing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost half of the world’s bird populations are declining (BirdLife International 2022) and migratory birds are declining faster than other groups (e.g. Laaksonen and Lehikoinen 2013; Runge et al. 2015). Migratory birds move vast distances annually between their breeding and non-breeding grounds to make use of the most suitable habitats and resources year-round (Alerstam et al. 2003). However, this makes them especially susceptible to changes in environmental conditions throughout their annual cycle (Ponti et al. 2020). Long-distance migrants especially spend only a short part of the annual cycle on their breeding grounds, thus uncovering factors that affect their survival during the non-breeding season provides crucial information needed to focus conservation efforts (Marra et al. 2015). Climatic conditions experienced during one part of the annual cycle can affect multiple demographic measures including individual survival (Newson et al. 2009; Pearce-Higgins et al. 2015). For example, precipitation levels and vegetation productivity on the non-breeding grounds have been associated with breeding population sizes of many migrant bird species (e.g. Ockendon et al. 2014; Schaub et al. 2005), although interpretation of the evidence has not always been straightforward (e.g. Beresford et al. 2019; Ockendon et al. 2014; Salewski et al. 2013). Indeed, estimating constant non-breeding site environmental conditions at large spatial scales may mask potential fine-scale differences in conditions experienced within regional non-breeding populations (Ishong et al. 2022).
Within populations, phenotypic differences may ultimately determine how environmental variables affect demographically important processes such as survival or sexual selection. In birds, breeding plumage coloration and quality can vary and lead to differential fitness among individuals (Dunn et al. 2015). Many bird species exhibit notable variation in colouration that can be explained by differences in genetic factors and in individual plasticity to environmental conditions (Roulin and Ducrest 2013). The existence of such variation indicates temporal and/or spatial changes in the direction of selection such as fluctuating selection for a specific phenotype (Bell 2010). This differential selection can lead to alternative phenotypes being associated with different habitats or to different abilities among phenotypes to cope with changing environmental conditions (Kassen 2002). For example, Gloger’s rule predicts that darker integuments are favoured in wetter (more humid) environments and lighter integument when conditions are drier (Gloger 1833). Evidence suggests that non-breeding habitat can have marked effects on individual fitness through carry-over effects (Marra et al. 1998; Norris et al. 2004; Reneerkens et al. 2020). Unfortunately, little is known about how different phenotypes are distributed within a species’ non-breeding range due to inherent difficulties in evaluating spatial migratory connectivity at fine spatial scales (Webster et al. 2002).
Measurements of naturally occurring stable isotopes in tissues have proven valuable in assessing migratory connectivity due to their ready application to each captured individual compared to the use of extrinsic markers such as GPS trackers that still pose substantial size and cost constraints (Hobson 1999a; Costa-Pereira et al. 2022), and which rarely can be used to infer local conditions mediated by diet. Tissue stable isotope measurements also provide a proxy for habitat characteristics during the period of tissue formation (Hobson 1999a). In particular, the stable isotopes of carbon (δ13C), nitrogen (δ15N), and hydrogen (δ2H) measured in feathers of birds provide useful information on moult origins and conditions as these isotopes are associated with vegetation types and land use as well as predictable spatial isotopic distributions or ‘isoscapes’ and all of them are expected to increase in drier conditions (Kelly 2000; Hobson et al. 2012b; Hoenig et al. 2021). In terrestrial systems, values of δ13C differentiate between primary producers with C3, C4 or CAM photosynthetic pathways (Bender 1968). Nitrogen stable isotope ratios (δ15N) are useful indicators of trophic position as they typically increase in a stepwise fashion with increasing trophic level (Hobson and Welch 1992; Hobson et al. 1994). Stable nitrogen isotope values can also be influenced by land-use practices and the use of fertilizers (Hobson 1999b; Hoenig et al. 2021). Tissue δ2H values are strongly associated with precipitation δ2H values, indicating connection between δ2H values and local rainfall (Hobson et al. 2012b). Thus, such isotopic markers in feathers can be used to infer provenance and act as an indicator of mesic vs. xeric conditions during feather growth (Marra et al. 1998; Hobson et al. 2012b; Vander Zanden et al. 2016; López-Calderón et al. 2017; van Wijk et al. 2021).
We investigated linkages between breeding plumage darkness and spatial origins and inferred environmental conditions on the non-breeding grounds in an Afro-Palaearctic migratory passerine, the pied flycatcher (Ficedula hypoleuca), by sampling feathers grown on their African non-breeding grounds. The pied flycatcher is a sexually dimorphic, insectivorous migratory passerine that undergoes a complete moult on the breeding grounds before autumn migration when males also change into a more cryptic, female-like plumage. A partial prenuptial moult occurs before spring migration in Africa when males moult into their conspicuous breeding plumage (Salewski et al. 2004; Jenni and Winkler 2020) with extensive variation in breeding plumage traits among males (Laaksonen et al. 2015). The colour of the dorsal plumage varies from almost completely black to almost completely brown closely resembling female Ficedula flycatchers breeding in sympatry (heterospecific mimicry; Calhim et al. 2014). Blackness in male plumage in breeding populations increases with increasing distance to Central Europe, while Central European males are mostly brown (Laaksonen et al. 2015). The dorsal colour is derived from melanin pigments which are strongly genetically regulated (Roulin and Ducrest 2013). The dorsal coloration of male pied flycatcher is heritable (Lehtonen et al. 2009) as well as highly repeatable within individuals (Järvistö et al. 2016). Males, regardless of colour, tend to become slightly darker after occupying dry non-breeding site conditions (Järvistö et al. 2016). While the conspicuous black plumage has been suggested to be sexually selected (Røskaft and Järvi 1983), evidence is mixed and instead it seems that fluctuating selection induced by changing temperatures during breeding maintain differently coloured pied flycatcher males in sympatry (Sirkiä et al. 2010; reviewed in Sirkiä and Qvarnström, 2021). Recently, Selonen et al. (2021) and Nater et al. (2022) showed using long-term datasets that large-scale population dynamics of pied flycatchers breeding in Finland and Great Britain are largely dependent on conditions experienced over the non-breeding period, therefore calling for the focus of studies and conservation efforts on migration routes and stationary non-breeding (hereafter, wintering) areas.
We used feathers collected annually as a part of a long-term study at a breeding site in Finland to infer the local conditions during moult in Africa in the full range of differently coloured individuals spanning eight years. We used stable isotope ratios (δ13Cf, δ15Nf, δ2Hf) of winter-grown feathers as a proxy of local, isotopically different habitats in Africa. Our objectives were: (1) to investigate potential differences in wintering-site use among male pied flycatchers varying in plumage coloration (i.e. blackness), and (2) to model effects of local wintering site and more general African winter conditions on the return probability of differently coloured males to the breeding grounds. We predicted that (1) blacker individuals would show lower isotope values indicating mesic habitats (Gloger 1833; Salewski et al. 2002b; Roulin 2004; Hobson et al. 2012b), and (2) individuals exhibiting lower isotope values and/or blacker plumage would show higher return rates than individuals showing higher isotope values, especially after overall dry winters (Järvistö et al. 2016) due to differences in rainfall across sites (Hobson et al. 2012b).
Materials and methods
Data collection
Feather samples were collected from a pied flycatcher population breeding on the island of Ruissalo in Turku, Finland during the years 2007–2014. The long-term work and sampling were approved by the Animal Experiment Board in Finland (LOS-2007-L-264-254; ESAVI-2010-05480/Ym-23). The study site included 230 nest boxes before 2011, after which the area was expanded to 436 nest boxes (inner bottom area: 144 cm2, entrance hole Ø: 32 mm) while maintaining the same sites in the original area. In Finland, pied flycatchers arrive to the breeding grounds in early May (Velmala et al. 2015) and males were captured between 02 May and 20 July as part of a long-term monitoring study. All birds were fitted with a uniquely numbered aluminium ring and aged either as second calendar year (i.e. 1-year old) or older (≥ 2 years) based on feather characteristics (Svensson 1992). The middle tertial feather from one wing was collected from each bird and used later for stable isotope analyses. This feather is moulted during the pre-nuptial moult on the wintering grounds in Africa (Svensson 1992; Salewski et al. 2004). The same feather was collected and same region of the feather analysed for stable isotopes in all cases (Smith et al. 2008). In each year, ~ 30 males (28 males in 2013; 29 males in 2012; 30 males in 2007, 2008, 2014; 31 males in 2009, 2010, 2011; 240 samples in total) representing a range in plumage colouration from fully brown to fully black were picked for feather stable isotope analyses. We note that the selected individuals do not to represent the phenotype distribution of the population, which varies annually (Sirkiä et al. 2013), since for the purpose of this study the idea was to have a balanced sample of the coloration range in each year. Colouration of each individual was visually estimated at the field as the approximate proportion of black feathers in the dorsal plumage (areas in the head and back excluding the rump) and reported in percentages from 0 to 100% (Järvistö 2016) in 5% intervals (with 1% exceptions in both extremes). Colouration assessments were done by several people over the years. However, new people were always trained by an experienced investigator to assess colouration, and the repeatability of separate colour measurements have previously been found to be high (r = 0.88, P < 0.001 in (Järvistö et al. 2015)). Thus, we have no reason to believe that the colour measurements in this study would be strongly influenced by the assessor. We also assumed that any isotopic differences that may be linked to differential melanin content in feathers were relatively minor compared to differences anticipated among xeric vs mesic habitats in Africa. Evidence for this assumption was provided by Michalik et al. (2010) who found minor isotopic effects in feathers for δ13C and δ15N values, but, to our knowledge, similar investigations have not been performed for δ2H values. Nonetheless, the work of Hobson et al. (2012b) using feathers from numerous species in North America with varying feather coloration has shown a strong influence of local precipitation vs any species/colouration effects.
Environmental variables
Three indices of environmental conditions (NAO, NDVI, and rainfall) were used to examine the relationships between feather characteristics and general annual wintering conditions. Finnish pied flycatcher wintering locations were estimated to be in West Africa between 5.5°N and 11.5°N, and 6.5°W and 15.5°W based on ring recoveries and geolocation data (n = 3 and n = 4, respectively) (Ouwehand et al. 2016). NDVI and rainfall values were calculated for terrestrial regions of the estimated wintering area as average values for February and March since pre-breeding moult peak of pied flycatchers occurs from mid-February to mid-March (Salewski et al. 2004). NAO (North Atlantic Oscillation index) values reflect climatic variation at a larger scale over terrestrial and marine areas in the Northern Hemisphere and winter NAO is routinely defined as average of values for December-March (Hurrell 1995; Jones et al. 1997). In Sub-Saharan and West Africa, negative NAO values correspond to wet winters and positive values to drier winters (Oba et al. 2001; Evan et al. 2006). NDVI (Normalized Difference Vegetation index) describes vegetation productivity of an area via remote sensing (Schmidt and Karnieli 2002). NDVI values range from of -1 to + 1 with higher values generally indicating greater productivity and more positive correlations with regional avian richness (Seto et al. 2004). The amount of rainfall was used as a third environmental index that directly reflects precipitation during the moulting period. NAO values were accessed at http://www.cru.uea.ac.uk/~timo/datapages/naoi.htm. Monthly means for NDVI and rainfall, available as 0.1° × 0.1° gridded rasters, were recorded by NASA Earth Observations (NEO) and were downloaded from http://neo.gsfc.nasa.gov.
Isotope measurements
Feathers were soaked in a 2:1 chloroform:methanol solution overnight, rinsed, and air dried under a fume hood for 24 h. For stable-carbon and nitrogen isotope analyses, we weighed 1 mg of feather into precombusted tin capsules. Encapsulated feather was combusted at 1030 °C in a Carlo Erba NA1500 or Eurovector 3000 elemental analyser. The resulting N2 and CO2 were separated chromatographically and introduced to an Elementar Isoprime or a Nu Instruments Horizon isotope ratio mass spectrometer. We used two reference materials to normalize the results to VPDB and AIR: BWBIII keratin (δ13C = − 20.18, δ15N = + 14.31‰, respectively) and PRCgel (δ13C = − 13.64‰, δ15N = + 5.07‰, respectively). Within run (n = 5) precisions as determined from both reference and sample duplicate analyses were ± 0.1‰ for both δ13C and δ15N.
Samples for stable hydrogen (δ2H) isotopes were weighed (0.35 mg) into silver capsules using the feather barbs only. Capsules were compressed and analysed using the LSIS-AFAR stable isotope facility at the University of Western Ontario. Samples were loaded into a Uni-prep carousel (Eurovector®, Milan, ITA) held at 60ºC, evacuated and maintained under positive pressure with dry helium and then combusted in a Eurovector 3000 elemental analyzer pyrolytically on glassy carbon at 1350ºC. Separated H2 was analyzed using a Thermo Delta V Plus (Thermo scientific®, Bremen, DEU) continuous-flow isotope ratio mass spectrometer via a Conflo device (Thermo Scientific®, Bremen, DEU). Sample results were expressed in the standard delta (δ) notation in parts per thousand (‰) deviation from the Vienna Standard Mean Ocean Water (VSMOW) standard. In-house keratin standards (CBS: -197‰; KHS: -54.1‰) were used in order to derive the δ2H value of the non-exchangeable H fraction according to the comparative equilibration approach (Wassenaar and Hobson 2003). Based on within-run (n = 5 each) keratin standards, measurement error was estimated to be ± 2‰.
Determining probable moult origins
To assess if moult origins of male pied flycatchers varied with blackness, we used a dual-isotope multivariate normal probability density function (mvnpdf) method described in detail elsewhere (Hobson et al. 2014). In brief, we conducted probabilistic assignment to origin analyses using δ2Hf and δ13Cf restricted to possible moult origins in the western part of the pied flycatcher African non-breeding range. We excluded δ15Nf from the assignments due to difficulties in modelling this isotope spatially because of the likely influence of agricultural inputs. We conducted assignments separately for pied flycatchers with low (< 33%), moderate (33–66%) and high (> 66%) blackness values (Fig. 1 in Online Resource 2). The multi-isotope mvnpdf approach assumes that the isoscapes are independently governed by different biogeoclimatic processes and therefore exhibit spatial non-stationarity. We first converted an amount-weighted mean growing-season precipitation δ2H (δ2Hp) isoscape surface (Bowen et al. 2005) to a feather isoscape using the calibration equation for known-origin migrant songbirds from Hobson et al. (2012a): δ2Hf = − 6.77 + 1.42* δ2Hp. We used a δ13C isoscape representing the theoretical spatial distribution of δ13C values in plants in Africa, which is based on annual plant δ13C composition approximately corresponding to mean annual conditions (Still and Powell 2010) and applied + 2‰ to the δ13C isoscape to account for discrimination between plants and herbivorous insects in feather isotopes. We assumed that plant-based isoscapes exhibit minimal annual changes in δ13C and so this isoscape provided the most current and accurate approximation of plant δ13C composition available for Africa.
Following the mvnpdf analysis, we used a conservative odds ratio to assign feathers to potential moult origin using the spatially explicit probability densities for individual samples where georeferenced locations (i.e. raster cells) with ≥ 66.7% probability was coded as potential origins (1) and all other locations (i.e. < 66.7%) were considered as improbable origins (0). Assignment to origin analyses conducted for each sample resulted in a spatially referenced binary raster file for each individual, which were subsequently summed across assignments for all individuals to represent potential origins in each blackness grouping. Assignment to origin analyses including spatial file manipulation were conducted using the ‘rgeos’, ‘mvtnorm’,’sf’, ‘sp’ and ‘Rfast’ packages in the R v4.1.1 computing environment (Genz and Bretz 2009; Bivand et al. 2013; Pebesma 2018; Bivand and Rundel 2023; Papadakis et al. 2023; Pebesma and Bivand 2023).
Statistical analyses
First, the relationships between plumage blackness and each stable isotope value were tested with a linear mixed model using percent blackness as the response variable and δ13Cf, δ15Nf, or δ2Hf values as individual explanatory variables. Age (young or old) was also included in the model as a fixed effect to control for possible age effects, as male pied flycatcher plumage tends to slightly darken (ca. 10–15%) between the ages 1 and 2 years (Lundberg and Alatalo 1992). Individual ring number was included as a random effect as some individuals (n = 31) were measured more than once. In this dataset, the year of feather collection by default did not explain any variance in plumage blackness, as individuals from each year were selected to represent a similar continuum from brown to black plumage (Fig. 2 in Online resource 2). Thus, year was not included in this analysis. Links between feather isotope values and the environmental variables were also explored to connect yearly local wintering conditions to general annual wintering conditions (Online resource 1).
Second, we modelled the local return probability of male pied flycatchers as a function of their colouration, local wintering conditions (feather isotope values) and general wintering conditions (environmental indices for the winter prior to return). Mixed effects Cox regressions were used for this analysis because of repeated individual measurements in the data and performed with functions ‘coxme’ and ‘Surv’ from the packages ‘coxme’ and ‘survival’ (Therneau 2022, 2023). Separate models were run for each isotope × environmental variable interactions (9 models) against the binary response variable (returned or not) including age at first capture (young or old) as a fixed effect. Individual identification was used as a random effect. Similar models were run for interactions between plumage coloration and environmental variables (3 models).
Our study suffers from an inherent limitation of not being able to link the wintering habitat of a specific year to the return rate of the same year as we lacked relevant feathers, from which to measure the isotope values from those individuals that did not return. However, Salewski et al. (2000) reported previously that almost a quarter (23.4%) of pied flycatchers that were captured in one wintering site in Africa returned to the exact same site in the following years. Thus, we used our stable isotope values as a proxy for conditions experienced by the individual also in the coming years. This approach assumes that the isotope values are repeatable within individuals. Thus, to determine whether birds returned to similar winter sites in different years, within-individual repeatabilities were calculated with each isotope as a response variable and individual ring number as a random effect using the function ‘rpt’ from the package ‘rptR’ (Stoffel et al. 2017).
All linear mixed models were run using the function ‘lmer’ in the package ‘lme4’ (Bates et al. 2015) and estimated using the restricted maximum likelihood method. Statistical significances for explanatory variables were obtained using the package ‘lmerTest’ (Kuznetsova et al. 2017). Degrees of freedom for fixed factors were calculated and parameter estimates, and their standard errors were assessed using the Kenward-Roger method with the package ‘pbkrtest’ (Halekoh and Højsgaard 2014). Statistical significance (α) was set at 0.05. All statistical analyses were conducted with R version 4.1.2 (R Core Team 2023).
Results
Both δ13Cf and δ15Nf values were highly repeatable within individuals across years (C: 0.685 (95% CI [0.50, 0.82], P < 0.001); N: 0.804 (95% CI [0.68, 0.90], P < 0.001)). In contrast, δ2Hf values were not repeatable (H: 0.0 (95% Cl [0, 0.38], p = 0.5)) (Fig. 3 in Online resource 2), which was expected, as δ2Hf values are linked with rainfall that varies among years. Local between-year changes in rainfall amount occur and likely result in low repeatability in δ2Hf values, while differences in average rainfall across multiple regions are less likely to change (Mohr 2004). Furthermore, male plumage colour was repeatable within individuals across years (R = 0.7 (95% CI [0.64, 0.76], P < 0.0001) in a larger sample of this population as reported in Järvistö et al. (2016), and R = 0.59 (95% CI [0.34, 0.78], P = 0.001) in these data for the 31 repeated individuals).
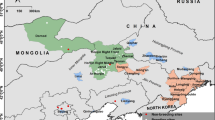
Male pied flycatcher plumage blackness was negatively related with δ2Hf values but not significantly related with δ13Cf or δ15Nf values (Table 1, Fig. 1). Browner males had higher δ2Hf values while blacker males exhibited, on average, lower δ2Hf values indicative of mesic habitats (Fig. 1a). To examine the variation of δ2Hf values across the colouration gradient we ran a separate model regressing δ2Hf values against the blackness index and regressed the residual absolute values of this model again against blackness. According to this analysis, there was non-significant (p = 0.15), positive relationship between blackness and variation in δ2Hf values (Fig. 4 in Online resource 2). Male pied flycatchers appeared to originate (i.e. had isotopic profiles that aligned with the underlying isoscapes) from generally similar regions in the southern part of their non-breeding range from Liberia to Nigeria regardless of blackness (Fig. 2, Fig. 1 in Online resource 2). However, assignment analyses showed a potential regional distinction between moulting sites of browner and blacker males; individuals with blackness values < 33% seem to have moulted in areas concentrated in eastern Liberia, southern Ivory Coast, western Ghana and southern Nigeria whereas individuals with blackness values > 33% had potential origins in more western areas across Liberia in addition to the regions of origin similar to birds with < 33% blackness (Fig. 2).
Associations between plumage blackness (in %) and feather isotope values (a hydrogen, b carbon, and c nitrogen in ‰) of individual pied flycatcher males. Significant association is indicated with black regression line, and non-significant (p = 0.08) tendency in b with grey regression line. In the analysis, dorsal plumage blackness is used as the response variable, but is presented here on the x-axis for illustration purposes
Depictions of purported non-breeding moult origins of male pied flycatchers with different blackness values (a < 33% blackness, N = 40; b 33–66% blackness, N = 77; c > 66% blackness, N = 119) assigned to the western part of their African non-breeding range d based on similarity in isotope values to the underlying isoscapes using a multivariate probability density function (see “Materials and methods”). Values in the legend indicate the minimum and maximum number of individuals potentially originating from a particular cell in the dual δ 2Hf and δ 13Cf isoscape
Across years, low δ2Hf values, but not δ13Cf nor δ15Nf values were linked to higher precipitation winters (Online resource 1, and Fig. 5 in Online resource 2). Values of δ2Hf, indicative of different local environmental conditions, were associated with plumage blackness in the cross-sectional sample and so we tested whether a change in winter conditions influenced the colour change in breeding plumage between years at the individual level. Using individuals with repeated samples, the between-year change in plumage colour was tested against the between-year change in δ2Hf value while controlling for age, but no relationship was found (β = 0.21, se = 0.45, t = 0.46, p = 0.65).
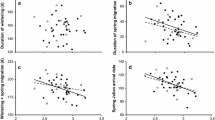
Values of δ13Cf and δ15Nf or their interactions with environmental indices did not influence the return probability of male pied flycatchers to the breeding grounds (Table 2). However, δ2Hf values had a significant interaction with both NAO (Table 2, Fig. 3a) and rainfall (Table 2, Fig. 3b) where individuals with low δ2Hf values were more likely to return when NAO was higher, and rainfall was lower the following winter (i.e. winter prior to their return). Despite the lack of within-individual repeatability in δ2Hf values as reported above, which hampers the use of single δ2Hf measurements to infer future measures, plumage colour is repeatable within individuals. Consequently, as δ2Hf values were negatively associated with plumage blackness, a similar trend with return rate was found for the interaction between plumage coloration and NAO (Blackness × NAO: β = 0.007, se = 0.004, z = 1.88, p = 0.06, Fig. 3c) but not for plumage colouration and rainfall (Fig. 3d). Therefore, blacker individuals tended to return at a higher rate than browner individuals after high NAO winters, while browner individuals were more likely to return after low NAO winters. However, as we were unable to provide direct evidence on the association between δ2Hf values and return rate, we consider this evidence as circumstantial.
Male pied flycatcher probability of return as model predicted risk scores (> 1 = increased probability to return; < 1 = decreased probability to return) in the next breeding season as a function of feather hydrogen isotope values (left side figures) or plumage blackness in % (right side figures) for winters with different NAO indices (upper row) or monthly average rainfall (lower row) during feather formation. Model predictor lines for high NAO/Rainfall values are indicated by dotted line (2.08/68.5 mm), for average values by solid line (− 0.31/57.1 mm), and for low values by dashed line (− 2.71/41.1 mm). These values are 10%, 50%, and 90% quantiles of the data. Environmental indices are shown as categorical for illustration but were treated as continuous variables in the analyses
Discussion
As predicted, feather δ2H values, but not δ13C or δ15N values, were associated with plumage blackness in male pied flycatchers so that, on average, δ2Hf values declined with increasing plumage blackness. The individual change in δ2Hf values did not explain the change in plumage blackness across years, indicating that differently coloured males inhabited different environments rather than indicating that environment influenced plumage colouration. Similarly, consistent with Ouwehand et al. (2016), we found high repeatability of both δ13Cf and δ15Nf values suggesting that individuals tend to return to the same, or at least similar isotopic areas or habitats for the winter year after year. As expected, males with lower δ2Hf values were more likely to return to the same breeding site than males with higher δ2Hf values after dry winters but the effect was reversed when winters were wetter.
Associations between plumage colour and habitat
In Africa, pied flycatchers inhabit broadleaved forests, shrublands, and grasslands (BirdLife International 2022) that vary in rainfall (Salewski et al. 2002b). Higher feather δ2H values have previously been associated with lower regional rainfall (Hobson and Wassenaar 1996; Hobson et al. 2012b) consistent with the rainfall “amount effect” describing local precipitation δ2H (Clark and Fritz 1997). Plumage and feather characteristics are often influenced by habitat and conditions during feather formation (e.g., Saino et al. 2004; Eggers and Low 2014; Meillère et al. 2017), and also pied flycatcher males have been shown to moult into darker plumage after drier winters (Järvistö et al. 2016). Here, we however show that individual change in plumage colouration did not follow the change in δ2H values indicating that local rainfall does not affect plumage coloration. This suggests that browner males tend to winter mainly in drier habitats while blacker males tend to be found in wetter areas. As there were no significant relationships between plumage coloration and feather δ13C or δ15N values, it appears that pied flycatchers overwinter in otherwise isotopically similar areas but that differ in the amount of annual precipitation. However, there was a tendency for δ13Cf values to decline with increasing plumage darkness, also indicating that browner individuals overwinter in drier habitats. Plants with a C3 photosynthetic pathway respond to heat and water stress by reducing stomatal openings thereby increasing their δ13C values. Our data underline the fact that local overwinter habitats consisted of C3 and C4 plants that represent long-term climatic averages (where food webs remain relatively constant in average δ13C) whereas rainfall amount is expected to be more variable among years and more directly linked to year-specific food web δ2H values (but see Vander Zanden et al. 2015). The broad likely wintering regions recognised by the assignment analysis align with wintering areas of the pied flycatcher identified using light-level geolocators, which showed moderate within-population connectivity (Ouwehand et al. 2016). While we cannot rule out that individuals with different blackness values overwinter in different regions based on this analysis, it is equally possible that these individuals use different habitats as described above.
Although wintering pied flycatcher males seem to distribute according to Gloger’s rule (Gloger 1833), the rule likely cannot be applied to the overwinter period because all pied flycatcher males are brown on their wintering grounds and only become darker after moult shortly before spring migration (Lundberg and Alatalo 1992; Svensson 1992). Instead, differences in individual competitive ability might influence the capacity to occupy and moult in different quality habitats in winter (Salewski et al. 2002b; Reudink et al. 2009). As insect abundance increases with rainfall (Sinclair 1978; Studds and Marra 2007), wetter habitats in Sub-Saharan Africa are arguably better habitat than drier areas for insectivorous passerines (López-Calderón et al. 2017). Previous research showed that pied flycatchers hold wintering site territories (Salewski et al. 2002a) and good competitive ability helps them acquire a good territory with more rainfall. The lowest δ2Hf values reflecting a wetter territory were found only in darker male pied flycatchers. Generally, darker melanin-based coloration is linked to aggressive behaviour through pleiotropic effects of the genes responsible for melanin production (Ducrest et al. 2008). Indeed, at the breeding grounds, darker males exhibit more territorial activity than lighter males (Slagsvold and Lifjeld 1988). Early evidence suggests that they also acquire better, more deciduous breeding territories than lighter males (Järvi et al. 1987). However, later studies may have obscured the potential link between plumage colour and breeding territory quality, potentially by offering a surplus of good nesting territories (Lundberg and Alatalo 1992; Silverin 1998) and links between plumage colour, aggressiveness, and competitive ability in this species have been similarly inconsistent (Järvi et al. 1987; Breiehagen and Sætre 1992; Huhta and Alatalo 1993).
The reproductive success of differently coloured pied flycatcher males depends on weather during different stages of breeding (Sirkiä et al. 2010; Järvistö et al. 2015), suggesting that differently coloured pied flycatchers could be adapted to different conditions, at least during the breeding season. Within-species adaptations to distinctive non-breeding habitats have been reported with other bird species, but these differences were attributed to morphology-induced differences in foraging strategy (Satgé et al. 2022) and personality (Chyb et al. 2021) rather than phenotypic differences. However, it is possible that differently coloured pied flycatchers occupy different wintering habitats because they favour different environmental conditions (Galeotti and Rubolini 2003; Roulin 2004; Forsman and Åberg 2008), potentially through the pleiotropic effects of melanin production and/or other genetic correlations (Ducrest et al. 2008; McKinnon and Pierotti 2010).
Climate, plumage and return rate
Individuals that wintered in areas with more rainfall (as indicated by lower feather δ2H values) were more likely to return to the same breeding site when the winter prior to returning was overall drier than average. While NAO represents a general global climatic index, different areas vary in mean rainfall within years (Jones et al. 1997; Mohr 2004). Therefore, in high NAO years, areas with higher-than-average rainfall more likely still receive some rain while other areas might suffer from drought. Interestingly, the return probability of individuals that wintered in wetter areas decreased with decreasing NAO and increasing rainfall. While sufficient rain likely increases survival over the non-breeding season and/or contributes to achieving good conditions for return migration (Marra et al. 1998; Rockwell et al. 2017), heavy rainfall can decrease the activity of aerial insects leading to reduction in feeding opportunities of insectivores (Veistola et al. 1997; Cowley and Siriwardena 2005) which consequently could lead to lowered individual body condition and insufficient preparation for migration.
As browner individuals winter more in drier habitats compared to blacker individuals, the weak positive connection between plumage blackness and return rate after high NAO winters was not surprising. Using a larger dataset from the whole population, Järvistö et al. (2016) found that the proportion of blacker individuals in the Ruissalo breeding population increased after high NAO winters corresponding to drier conditions. Overall dry winters likely benefit blacker males occupying locally wetter areas as poor wintering conditions can hinder fuelling for migration which may delay migration departure and subsequently lead to later spring arrival date (Marra et al. 1998; Gunnarsson et al. 2006; Ouwehand and Both 2017). In the case of a nest box population of pied flycatchers, this could result in late males settling outside the study area in often poor quality natural cavities (Lundberg and Alatalo 1992), which would leave them out of the breeding population monitoring. When environmental conditions in northern Central Europe are favourable during northward migration, the proportion of browner males in our breeding population increased, indicating a prolonged migration of Central European brown male pied flycatchers (Sirkiä et al. 2013). Conversely, unfavourable environmental or poor individual conditions might shorten the spring migration of browner individuals so that they do not return to the previous, more northern breeding sites, but this hypothesis remains to be studied.
Our study revealed an association between inferred habitat quality on African wintering grounds and the return rate of differently coloured male pied flycatchers to Finland. This contrasts with the previously held notion that breeding plumage colour is not related to overwinter survival, as indicated by return rates (Lundberg and Alatalo 1992). Return rates of pied flycatcher males were investigated in the 1980s and early 1990s in Scandinavia and Spain, where males were classified as black or brown, and depending on the year, either higher return rates of brown or black males, or no differences between the classes were found (Røskaft et al. 1986; Slagsvold and Lifjeld 1988; Potti and Montalvo 1991; Alatalo et al. 1994). Interestingly, winter NAO values across these study periods varied greatly (1981–91 values from -0.38 to 2.86, average of 0.85). Especially high NAO values preceded the breeding seasons in 1983 and 1989 (NAO = 2.00 and 2.86, respectively, cf. 2.08, the highest NAO value within current study period), when higher return rates of black males to the breeding grounds were also observed, similar to our study. Male plumage colouration has strong effects on the breeding success of the breeding pair, but in a temperature related manner where nestling mortality of blacker males is higher than browner males when it is cold (Sirkiä et al. 2010), and blacker males produce heavier fledglings when it is warm during the nestling period, but lighter fledglings than those of browner males when it is cold (Järvistö et al. 2015). Thus, mismatch between wintering and breeding conditions (i.e. wintering conditions favouring one colour morph but breeding conditions the other) would likely have dire consequences for the breeding success of the population. As far as we know, no study has investigated direct links between wintering conditions and subsequent breeding success in differently coloured male pied flycatchers. Teerikorpi et al. (2018) found that after high NAO, thus drier winters, male pied flycatchers with larger white wing patches attracted females that laid larger clutches which also had better local survival to the following breeding seasons, while the effect was reversed after low NAO winters. White wing patches have been previously shown to get smaller during high NAO winters, while large wing-patched males had higher return rates to the breeding population than small wing-patched males after drier winters. This pattern was reversed after moister winters (Järvistö et al. 2016), similarly as in the current study in relation to winter NAO and black plumage colouration. These results together elaborate the influence of wintering conditions on phenotypic compositions of breeding populations, and ultimately breeding success through return rates and male quality in the breeding environment.
Conclusions
Global climate change is altering local environments experienced by different species. Genetic diversity, as reflected in degree of colour polymorphism, can increase the resilience of a species against climate change, but one morph might be favoured over others (Roulin 2014). Indeed, observed changes in phenotypic abundance within populations can indicate environmental changes over large temporal scales (Karell et al. 2011). Stable isotope measurements in feathers are an important proxy for winter habitat use on the moulting grounds. Across Europe, pied flycatcher populations with mostly brown males have experienced more dramatic declines in past decades than populations with both browner and blacker males (Both et al. 2006; Lehikoinen and Piha 2021; Nater et al. 2022). Using a breeding population consisting of male pied flycatchers of both colour extremes, our isotope study combined with NAO index showed that browner males winter in drier areas more than blacker males, and have reduced return rates after overall drier winters. If this pattern also holds true in other populations, the brown morph of the pied flycatcher may be at risk of declining as melanin-coloration is heritable, and according to climate change projections, drier parts of West Africa are expected to get even drier, while the moist eastern parts are expected to experience more, and heavier, rainfall (Trisos et al. 2022). Morph-specific population declines reduce the genetic diversity of a species, rendering it less resilient against further changes in climatic conditions. Future studies should therefore investigate the wintering conditions of other brown males, but also of females, which are always brown and fundamentally important for the resilience of a species. Changing conditions likely affect not only pied flycatchers but other Afrotropical migrant species wintering in the same areas. In addition to changing climate, alteration of wintering habitats due to increased deforestation related to intensified agriculture and grazing, are important drivers of declining breeding population trends of European migrants wintering in West Africa (Howard et al. 2020). Effective conservation of long-distance migrant birds, such as the pied flycatcher, thus would require targeted land management actions in Africa that ensure conservation of suitable wintering areas while considering of future climate change scenarios.
Data availability
Data used in this study are available in Figshare (https://doi.org/10.6084/m9.figshare.25674441).
References
Alatalo RV, Gustafsson L, Lundberg A (1994) Male coloration and species recognition in sympatric flycatchers. Proc R Soc Lond B 256:113–118. https://doi.org/10.1098/rspb.1994.0057
Alerstam T, Hedenström A, Åkesson S (2003) Long-distance migration: evolution and determinants. Oikos 103:247–260. https://doi.org/10.1034/j.1600-0706.2003.12559.x
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bell G (2010) Fluctuating selection: the perpetual renewal of adaptation in variable environments. Philos Trans R Soc B Biol Sci 365:87–97. https://doi.org/10.1098/rstb.2009.0150
Bender MM (1968) Mass spectrometric studies of carbon 13 variations in corn and other grasses. Radiocarbon 10:468–472. https://doi.org/10.1017/S0033822200011103
Beresford AE, Sanderson FJ, Donald PF et al (2019) Phenology and climate change in Africa and the decline of Afro-Palearctic migratory bird populations. Remote Sens Ecol Conserv 5:55–69. https://doi.org/10.1002/rse2.89
BirdLife International (2022) State of the World’s Birds 2022—BirdLife International. https://www.birdlife.org/papers-reports/state-of-the-worlds-birds-2022/. Accessed 8 Nov 2022
Bivand R, Rundel C (2023) rgeos: Interface to Geometry Engine - Open Source ('GEOS’)
Bivand RS, Pebesma E, Gómez-Rubio V (2013) Applied spatial data analysis with R. Springer, New York
Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in a long-distance migratory bird. Nature 441:81–83. https://doi.org/10.1038/nature04539
Bowen GJ, Wassenaar LI, Hobson KA (2005) Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 143:337–348. https://doi.org/10.1007/s00442-004-1813-y
Breiehagen T, Sætre G-P (1992) Territorial defence and plumage colour in pied flycatchers, Ficedula hypoleuca. Anim Behav 44:987–989. https://doi.org/10.1016/S0003-3472(05)80595-0
Calhim S, Adamik P, Järvistö P et al (2014) Heterospecific female mimicry in Ficedula flycatchers. J Evol Biol 27:660–666. https://doi.org/10.1111/jeb.12328
Chyb A, Jedlikowski J, Włodarczyk R, Minias P (2021) Consistent choice of landscape urbanization level across the annual cycle in a migratory waterbird species. Sci Rep 11:836. https://doi.org/10.1038/s41598-020-80872-3
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press
Costa-Pereira R, Moll RJ, Jesmer BR, Jetz W (2022) Animal tracking moves community ecology: opportunities and challenges. J Anim Ecol 91:1334–1344. https://doi.org/10.1111/1365-2656.13698
Cowley E, Siriwardena GM (2005) Long-term variation in survival rates of Sand Martins Riparia riparia: dependence on breeding and wintering ground weather, age and sex, and their population consequences. Bird Study 52:237–251. https://doi.org/10.1080/00063650509461397
Ducrest A-L, Keller L, Roulin A (2008) Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol Evol 23:502–510. https://doi.org/10.1016/j.tree.2008.06.001
Dunn PO, Armenta JK, Whittingham LA (2015) Natural and sexual selection act on different axes of variation in avian plumage color. Sci Adv 1:e1400155. https://doi.org/10.1126/sciadv.1400155
Eggers S, Low M (2014) Differential demographic responses of sympatric Parids to vegetation management in boreal forest. For Ecol Manag 319:169–175. https://doi.org/10.1016/j.foreco.2014.02.019
Evan AT, Heidinger AK, Knippertz P (2006) Analysis of winter dust activity off the coast of West Africa using a new 24-year over-water advanced very high resolution radiometer satellite dust climatology. J Geophys Res Atmos. https://doi.org/10.1029/2005JD006336
Forsman A, Åberg V (2008) Variable coloration is associated with more northerly geographic range limits and larger range sizes in North American lizards and snakes. Evol Ecol Res 10:1025–1036
Galeotti P, Rubolini D (2003) The niche variation hypothesis and the evolution of colour polymorphism in birds: a comparative study of owls, nightjars and raptors. Biol J Lin Soc 82:237–248. https://doi.org/10.1111/j.1095-8312.2004.00355.x
Genz A, Bretz F (2009) Computation of multivariate normal and t probabilities. Springer Science & Business Media, Berlin
Gloger CWL (1833) Das Abändern der Vögel durch Einfluß des Klima’s: Nach zoologischen, zunächst von den europäischen Landvögeln entnommenen Beobachtungen dargestellt ... Schulz
Gunnarsson TG, Gill JA, Atkinson PW et al (2006) Population-scale drivers of individual arrival times in migratory birds. J Anim Ecol 75:1119–1127. https://doi.org/10.1111/j.1365-2656.2006.01131.x
Halekoh U, Højsgaard S (2014) A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models—the R Package pbkrtest. J Stat Softw 59:1–32. https://doi.org/10.18637/jss.v059.i09
Hobson KA (1999a) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326. https://doi.org/10.1007/s004420050865
Hobson KA (1999b) Stable-carbon and nitrogen isotope ratios of songbird feathers grown in two terrestrial biomes: implications for evaluating trophic relationships and breeding origins. Condor 101:799–805. https://doi.org/10.2307/1370067
Hobson KA, Wassenaar LI (1996) Linking breeding and wintering grounds of neotropical migrant songbirds using stable hydrogen isotopic analysis of feathers. Oecologia 109:142–148. https://doi.org/10.1007/s004420050068
Hobson KA, Welch HE (1992) Determination of trophic relationships within a high Arctic marine food web using δ 13 C and δ 15 N analysis. Mar Ecol Prog Ser 84:9–18
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798. https://doi.org/10.2307/5256
Hobson KA, Van Wilgenburg SL, Wassenaar LI et al (2012a) A multi-isotope (δ13C, δ15N, δ2H) feather isoscape to assign Afrotropical migrant birds to origins. Ecosphere 3:art44. https://doi.org/10.1890/ES12-00018.1
Hobson KA, Wilgenburg SLV, Wassenaar LI, Larson K (2012b) Linking hydrogen (δ2H) isotopes in feathers and precipitation: sources of variance and consequences for assignment to isoscapes. PLoS ONE 7:e35137. https://doi.org/10.1371/journal.pone.0035137
Hobson KA, Van Wilgenburg SL, Wesoowski T et al (2014) A multi-isotope (δ 2H, δ 13C, δ 15N) approach to establishing migratory connectivity in Palearctic-Afrotropical migrants: an example using wood warblers Phylloscopus sibilatrix. Acta Ornithologica 49:59–71. https://doi.org/10.3161/000164514X682896
Hoenig BD, Snider AM, Forsman AM et al (2021) Current methods and future directions in avian diet analysis. Ornithology. https://doi.org/10.1093/ornithology/ukab077
Howard C, Stephens PA, Pearce-Higgins JW et al (2020) Disentangling the relative roles of climate and land cover change in driving the long-term population trends of European migratory birds. Divers Distrib 26:1442–1455. https://doi.org/10.1111/ddi.13144
Huhta E, Alatalo RV (1993) Plumage colour and male-male interactions in the pied flycatcher. Anim Behav 45:511–518. https://doi.org/10.1006/anbe.1993.1062
Hurrell JW (1995) Decadal trends in the North Atlantic oscillation: regional temperatures and precipitation. Science 269:676–679. https://doi.org/10.1126/science.269.5224.676
Ishong JA, Afrifa JK, Iwajomo SB et al (2022) Population trends of resident and migrant West African bird species monitored over an 18-year period in central Nigeria. Ostrich 93:171–186. https://doi.org/10.2989/00306525.2022.2068691
Järvi T, Røskaft E, Bakken M, Zumsteg B (1987) Evolution of variation in male secondary sexual characteristics. Behav Ecol Sociobiol 20:161–169. https://doi.org/10.1007/BF00299729
Järvistö PE (2016) Abiotic and biotic effects on secondary sexual traits at the population and individual levels in a passerine bird
Järvistö PE, Calhim S, Schuett W et al (2015) Foster, but not genetic, father plumage coloration has a temperature-dependent effect on offspring quality. Behav Ecol Sociobiol 69:335–346. https://doi.org/10.1007/s00265-014-1846-0
Järvistö PE, Calhim S, Schuett W et al (2016) Carry-over effects of conditions at the wintering grounds on breeding plumage signals in a migratory bird: roles of phenotypic plasticity and selection. J Evol Biol 29:1569–1584. https://doi.org/10.1111/jeb.12892
Jenni L, Winkler R (2020) Moult and ageing of European Passerines, 2nd edn. Bloomsbury Publishing, London
Jones PD, Jonsson T, Wheeler D (1997) Extension to the North Atlantic oscillation using early instrumental pressure observations from Gibraltar and south-west Iceland. Int J Climatol 17:1433–1450. https://doi.org/10.1002/(SICI)1097-0088(19971115)17:13%3c1433::AID-JOC203%3e3.0.CO;2-P
Karell P, Ahola K, Karstinen T et al (2011) Climate change drives microevolution in a wild bird. Nat Commun 2:208. https://doi.org/10.1038/ncomms1213
Kassen R (2002) The experimental evolution of specialists, generalists, and the maintenance of diversity. J Evol Biol 15:173–190. https://doi.org/10.1046/j.1420-9101.2002.00377.x
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27. https://doi.org/10.1139/z99-165
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Laaksonen T, Lehikoinen A (2013) Population trends in boreal birds: continuing declines in agricultural, northern, and long-distance migrant species. Biol Conserv 168:99–107. https://doi.org/10.1016/j.biocon.2013.09.007
Laaksonen T, Sirkiä PM, Calhim S et al (2015) Sympatric divergence and clinal variation in multiple coloration traits of Ficedula flycatchers. J Evol Biol 28:779–790. https://doi.org/10.1111/jeb.12604
Lehikoinen P, Piha M (2021) Sisämaan seurantapyynti 1987–2021: Yleisimpien varpuslintujen kannankehitys, poikastuotto ja elossasäilyvyys. Linnut Vuosikirja 2021:40–49
Lehtonen PK, Laaksonen T, Artemyev AV et al (2009) Geographic patterns of genetic differentiation and plumage colour variation are different in the pied flycatcher (Ficedula hypoleuca). Mol Ecol 18:4463–4476. https://doi.org/10.1111/j.1365-294X.2009.04364.x
López-Calderón C, Hobson KA, Marzal A et al (2017) Environmental conditions during winter predict age- and sex-specific differences in reproductive success of a trans-Saharan migratory bird. Sci Rep 7:18082. https://doi.org/10.1038/s41598-017-18497-2
Lundberg A, Alatalo RV (1992) The Pied Flycatcher. T. & A.D. Poyser, London
Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282:1884–1886. https://doi.org/10.1126/science.282.5395.1884
Marra PP, Cohen EB, Loss SR et al (2015) A call for full annual cycle research in animal ecology. Biol Lett 11:20150552. https://doi.org/10.1098/rsbl.2015.0552
McKinnon JS, Pierotti MER (2010) Colour polymorphism and correlated characters: genetic mechanisms and evolution. Mol Ecol 19:5101–5125. https://doi.org/10.1111/j.1365-294X.2010.04846.x
Meillère A, Brischoux F, Henry P-Y et al (2017) Growing in a city: consequences on body size and plumage quality in an urban dweller, the house sparrow (Passer domesticus). Landsc Urban Plan 160:127–138. https://doi.org/10.1016/j.landurbplan.2016.12.014
Michalik A, McGill RAR, Furness RW et al (2010) Black and white—does melanin change the bulk carbon and nitrogen isotope values of feathers? Rapid Commun Mass Spectrom 24:875–878. https://doi.org/10.1002/rcm.4462
Mohr KI (2004) Interannual, monthly, and regional variability in the wet season diurnal cycle of precipitation in sub-saharan Africa. J Clim 17:2441–2453. https://doi.org/10.1175/1520-0442(2004)017%3c2441:IMARVI%3e2.0.CO;2
Nater CR, Burgess MD, Coffey P et al (2022) Spatial consistency in drivers of population dynamics of a declining migratory bird. J Anim Ecol. https://doi.org/10.1111/1365-2656.13834
Newson SE, Mendes S, Crick HQP et al (2009) Indicators of the impact of climate change on migratory species. Endangered Species Res 7:101–113. https://doi.org/10.3354/esr00162
Norris DR, Marra PP, Kyser TK et al (2004) Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc R Soc Lond B 271:59–64. https://doi.org/10.1098/rspb.2003.2569
Oba G, Post E, Stenseth NC (2001) Sub-saharan desertification and productivity are linked to hemispheric climate variability. Glob Change Biol 7:241–246. https://doi.org/10.1046/j.1365-2486.2001.00405.x
Ockendon N, Johnston A, Baillie SR (2014) Rainfall on wintering grounds affects population change in many species of Afro-Palaearctic migrants. J Ornithol 155:905–917. https://doi.org/10.1007/s10336-014-1073-5
Ouwehand J, Both C (2017) African departure rather than migration speed determines variation in spring arrival in pied flycatchers. J Anim Ecol 86:88–97. https://doi.org/10.1111/1365-2656.12599
Ouwehand J, Ahola MP, Ausems ANMA et al (2016) Light-level geolocators reveal migratory connectivity in European populations of pied flycatchers Ficedula hypoleuca. J Avian Biol 47:69–83. https://doi.org/10.1111/jav.00721
Papadakis M, Tsagris M, Dimitriadis M, et al (2023) Rfast: a collection of efficient and extremely fast R functions
Pearce-Higgins JW, Eglington SM, Martay B, Chamberlain DE (2015) Drivers of climate change impacts on bird communities. J Anim Ecol 84:943–954. https://doi.org/10.1111/1365-2656.12364
Pebesma E (2018) Simple features for R: standardized support for spatial vector data. R J 10:439–446
Pebesma E, Bivand R (2023) Spatial data science. https://r-spatial.org/book/. Accessed 4 Mar 2024
Ponti R, Arcones A, Ferrer X, Vieites DR (2020) Seasonal climatic niches diverge in migratory birds. Ibis 162:318–330. https://doi.org/10.1111/ibi.12784
Potti J, Montalvo S (1991) Male colour variation in Spanish pied flycatchers Ficedula hypoleuca. Ibis 133:293–299. https://doi.org/10.1111/j.1474-919X.1991.tb04572.x
R Core Team (2023) R : A language and environment for statistical computing
Reneerkens J, Versluijs TSL, Piersma T et al (2020) Low fitness at low latitudes: wintering in the tropics increases migratory delays and mortality rates in an Arctic breeding shorebird. J Anim Ecol 89:691–703. https://doi.org/10.1111/1365-2656.13118
Reudink MW, Studds CE, Marra PP et al (2009) Plumage brightness predicts non-breeding season territory quality in a long-distance migratory songbird, the American redstart Setophaga ruticilla. J Avian Biol 40:34–41. https://doi.org/10.1111/j.1600-048X.2008.04377.x
Rockwell SM, Wunderle JM, Sillett TS et al (2017) Seasonal survival estimation for a long-distance migratory bird and the influence of winter precipitation. Oecologia 183:715–726. https://doi.org/10.1007/s00442-016-3788-x
Røskaft E, Järvi T (1983) Male plumage colour and mate choice of female Pied Flycatchers Ficedula hypoleuca. Ibis 125:396–400. https://doi.org/10.1111/j.1474-919X.1983.tb03129.x
Røskaft E, Järvi T, Nyholm NEI et al (1986) Geographic variation in secondary sexual plumage colour characteristics of the male pied flycatcher. Ornis Scand. (scand. J. Ornithol.) 17:293–298. https://doi.org/10.2307/3676816
Roulin A (2004) The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol Rev 79:815–848. https://doi.org/10.1017/S1464793104006487
Roulin A (2014) Melanin-based colour polymorphism responding to climate change. Glob Change Biol 20:3344–3350. https://doi.org/10.1111/gcb.12594
Roulin A, Ducrest A-L (2013) Genetics of colouration in birds. Semin Cell Dev Biol 24:594–608. https://doi.org/10.1016/j.semcdb.2013.05.005
Runge CA, Watson JEM, Butchart SHM et al (2015) Protected areas and global conservation of migratory birds. Science 350:1255–1258. https://doi.org/10.1126/science.aac9180
Saino N, Szép T, Ambrosini R et al (2004) Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proc R Soc Lond B 271:681–686. https://doi.org/10.1098/rspb.2003.2656
Salewski V, Bairlein F, Leisler B (2000) Recurrence of some palaearctic migrant passerine species in West Africa. Ringing Migr 20:29–30. https://doi.org/10.1080/03078698.2000.9674224
Salewski V, Bairlein F, Leisler B (2002a) Different wintering strategies of two Palearctic migrants in West Africa—a consequence of foraging strategies? Ibis 144:85–93. https://doi.org/10.1046/j.0019-1019.2001.00007.x
Salewski V, Falk KH, Bairlein F et al (2002b) A preliminary assessment of the habitat selection of two Palaearctic migrant passerine species in West Africa. Ostrich 73:114–118. https://doi.org/10.1080/00306525.2002.11446739
Salewski V, Altwegg R, Erni B et al (2004) Moult of three Palaearctic migrants in their West African winter quarters. J Ornithol 145:109–116. https://doi.org/10.1007/s10336-004-0020-2
Salewski V, Hochachka WM, Fiedler W (2013) Multiple weather factors affect apparent survival of European Passerine birds. PLoS ONE 8:e59110. https://doi.org/10.1371/journal.pone.0059110
Satgé YG, Keitt BS, Gaskin CP et al (2022) Temporal and spatial segregations between phenotypes of the Diablotin Black-capped Petrel Pterodroma hasitata during the breeding and non-breeding periods. bioRxiv. https://doi.org/10.1101/2022.06.02.491532
Schaub M, Kania W, Köppen U (2005) Variation of primary production during winter induces synchrony in survival rates in migratory white storks Ciconia ciconia. J Anim Ecol 74:656–666. https://doi.org/10.1111/j.1365-2656.2005.00961.x
Schmidt H, Karnieli A (2002) Analysis of the temporal and spatial vegetation patterns in a semi-arid environment observed by NOAA AVHRR imagery and spectral ground measurements. Int J Remote Sens 23:3971–3990. https://doi.org/10.1080/01431160110115780
Selonen V, Helle S, Laaksonen T et al (2021) Identifying the paths of climate effects on population dynamics: dynamic and multilevel structural equation model around the annual cycle. Oecologia 195:525–538. https://doi.org/10.1007/s00442-020-04817-3
Seto KC, Fleishman E, Fay JP, Betrus CJ (2004) Linking spatial patterns of bird and butterfly species richness with Landsat TM derived NDVI. Int J Remote Sens 25:4309–4324. https://doi.org/10.1080/0143116042000192358
Silverin B (1998) Territorial behaviour and hormones of pied flycatchers in optimal and suboptimal habitats. Anim Behav 56:811–818. https://doi.org/10.1006/anbe.1998.0823
Sinclair ARE (1978) Factors affecting the food supply and breeding season of resident birds and movements of palaearctic migrants in a tropical African Savannah. Ibis 120:480–497. https://doi.org/10.1111/j.1474-919X.1978.tb06813.x
Sirkiä PM, Qvarnström A (2021) Adaptive coloration in pied flycatchers (Ficedula hypoleuca)—the devil is in the detail. Ecol Evol 11:1501–1525. https://doi.org/10.1002/ece3.7048
Sirkiä PM, Virolainen M, Laaksonen T (2010) Melanin coloration has temperature-dependent effects on breeding performance that may maintain phenotypic variation in a passerine bird. J Evol Biol 23:2385–2396. https://doi.org/10.1111/j.1420-9101.2010.02100.x
Sirkiä PM, Virolainen M, Lehikoinen E, Laaksonen T (2013) Fluctuating selection and immigration as determinants of the phenotypic composition of a population. Oecologia 173:305–317. https://doi.org/10.1007/s00442-013-2593-z
Slagsvold T, Lifjeld JT (1988) Plumage colour and sexual selection in the pied flycatcher Ficedula hypoleuca. Anim Behav 36:395–407. https://doi.org/10.1016/S0003-3472(88)80010-1
Smith AD, Donohue K, Dufty AM Jr (2008) Intrafeather and intraindividual variation in the stable-hydrogen isotope (δD) content of raptor feathers. Condor 110:500–506. https://doi.org/10.1525/cond.2008.8515
Still CJ, Powell RL (2010) Continental-scale distributions of vegetation stable carbon isotope ratios. In: West JB, Bowen GJ, Dawson TE, Tu KP (eds) Isoscapes: understanding movement, pattern, and process on earth through isotope mapping. Springer Netherlands, pp 179–193
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Studds CE, Marra PP (2007) Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird, Setophaga ruticilla. Climate Res 35:115–122. https://doi.org/10.3354/cr00718
Svensson L (1992) Identification guide to European passerines. Märsta Press, Stockholm
Teerikorpi PE, Sirkiä PM, Laaksonen T (2018) Ecological crossovers of sexual signaling in a migratory bird. Evolution 72:2038–2048. https://doi.org/10.1111/evo.13515
Therneau TM (2022) coxme: mixed effects Cox models. R package version 22-181
Therneau TM (2023) A package for survival analysis in R. R package version 35-5
Trisos CH, Adelekan IO, Totin E, et al (2022) Africa. In: Pörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B, editors. Climate change 2022: impacts, adaptation, and vulnerability. contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 1285–1455
van Wijk RE, Barshep Y, Hobson KA (2021) On the use of stable hydrogen isotope measurements (δ2H) to discern trophic level in avian terrestrial food webs. Diversity 13:202. https://doi.org/10.3390/d13050202
Vander Zanden HB, Wunder MB, Hobson KA et al (2015) Space-time tradeoffs in the development of precipitation-based isoscape models for determining migratory origin. J Avian Biol 46:658–667. https://doi.org/10.1111/jav.00656
Vander Zanden HB, Soto DX, Bowen GJ, Hobson KA (2016) Expanding the isotopic toolbox: applications of hydrogen and oxygen stable isotope ratios to food web studies. Front Ecol Evol 4:20
Veistola S, Lehikoinen E, Eeva T (1997) Weather and breeding success at high latitudes—the pied flycatcher Ficedula hypoleuca and the Siberian tit parus cinctus. Ornis Fennica 74:89–98
Velmala W, Helle S, Ahola MP et al (2015) Natural selection for earlier male arrival to breeding grounds through direct and indirect effects in a migratory songbird. Ecol Evol 5:1205–1213. https://doi.org/10.1002/ece3.1423
Wassenaar LI, Hobson KA (2003) Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isot Environ Health Stud 39:211–217. https://doi.org/10.1080/1025601031000096781
Webster MS, Marra PP, Haig SM et al (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83. https://doi.org/10.1016/S0169-5347(01)02380-1
Acknowledgements
We thank Pauliina Teerikorpi for her influence in commencing this study and all the field assistants that assisted in the long-term monitoring work. Blanca Mora-Alvarez assisted with preparation of feathers for stable isotope analyses and Geoff Koehler assisted with isotope measurements.
Funding
Open Access funding provided by University of Turku (including Turku University Central Hospital). The study was financially supported by Ella and Georg Ehrnrooth Foundation and Emil Aaltonen foundation (grants to TK), and Academy of Finland (project 263651 to TL).
Author information
Authors and Affiliations
Contributions
TL led the long-term monitoring program during which the study materials were collected and conceived the study with Pauliina Teerikorpi. KAH performed stable H isotope analyses and coordinated all isotope laboratory work. TK analysed the data and KJK performed assignment analyses. TK wrote the manuscript with critical input from all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. The long-term work and sampling were approved by the Animal Experiment Board in Finland (LOS-2007-L-264-254; ESAVI-2010–05480/Ym-23).
Additional information
Communicated by Christian Voigt.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kärkkäinen, T., Hobson, K.A., Kardynal, K.J. et al. Winter-ground microhabitat use by differently coloured phenotypes affects return rate in a long-distance migratory bird. Oecologia 205, 163–176 (2024). https://doi.org/10.1007/s00442-024-05561-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-024-05561-8