Abstract
Parasitic infections are a global occurrence and impact the health of many species. Coinfections, where two or more species of parasite are present in a host, are a common phenomenon across species. Coinfecting parasites can interact directly or indirectly via their manipulation of (and susceptibility to) the immune system of their shared host. Helminths, such as the cestode Schistocephalus solidus, are well known to suppress immunity of their host (threespine stickleback, Gasterosteus aculeatus), potentially facilitating other parasite species. Yet, hosts can evolve a more robust immune response (as seen in some stickleback populations), potentially turning facilitation into inhibition. Using wild-caught stickleback from 20 populations with non-zero S. solidus prevalence, we tested an a priori hypothesis that S. solidus infection facilitates infection by other parasites. Consistent with this hypothesis, individuals with S. solidus infections have 18.6% higher richness of other parasites compared to S. solidus-uninfected individuals from the same lakes. This facilitation-like trend is stronger in lakes where S. solidus is particularly successful but is reversed in lakes with sparse and smaller cestodes (indicative of stronger host immunity). These results suggest that a geographic mosaic of host–parasite co-evolution might lead to a mosaic of between-parasite facilitation/inhibition effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most parasites co-occur with other parasites in a host (Telfer et al. 2010; Adegnika and Kremsner 2012; Thumbi et al. 2014). Coinfection of two or more parasites within an individual host creates the opportunity for species interactions between parasites. These interactions may be positive if the presence of one parasite species increases fitness of another parasite species (e.g., facilitation). Or interactions may be negative (inhibition) when the presence of one parasite species decreases fitness of other parasite species. These within-host interactions among parasites can lead to emergent changes in parasite transmission, abundance, and distributions (Griffiths et al. 2011). For example, in mice, the nematode Heligmosomoides polygyrus contributes toward facilitating survival of other parasites, such as Nippostrongylus brasiliensis, by inhibiting the host’s ability to expel them (Colwell and Wescott 1973). On the other end of the spectrum, mice infected with the nematode Trichinella spiralis have decreased parasitemia of the protozoan parasite Plasmodium berghei (Ngwenya 1982). To date, most analyses of inhibition/facilitation among parasites have been limited to laboratory experiments, though some field studies have also provided clear case examples (Ezenwa et al. 2010; see Hellard et al. 2015 for a review). Examples from aquatic systems are especially sparse.
Within-host interactions among parasites can arise through direct exchanges between parasites: physical contact, secretion of allelopathic chemicals, or cross-feeding (Turner et al. 1996; Balmer et al. 2009; Hammarlund et al. 2019). Alternatively, parasites can interact indirectly via mutual alteration of host traits such as immune activity or nutrient status (Råberg et al. 2006). For example, the inhibition of P. berghei parasitemia by T. spiralis in mice (mentioned above) may be a result of mononuclear phagocyte system activation and reduction in levels of reticulocytes (Ngwenya 1982). Nematodes infecting Cape buffalo induce a Th2 immune response, which inhibits the Th1 response needed against bacterial pathogens, thus facilitating bovine tuberculosis infection (Ezenwa et al. 2010; Jolles et al. 2008).
A key difference between direct and indirect interactions between parasites is that the latter, being mediated via changes in host traits, may be contingent on host genotype. As hosts evolve immunity to a given parasite, they may become recalcitrant to its immunosuppressive effects. As a result, in principle, the evolution of host immunity (or tolerance) might toggle a given parasite from a facilitative to inhibitory effect on its community members. When hosts are distributed in discrete populations that evolve independently, the geographic mosaic of co-evolution (Thompson 2004) may mean that one parasite species is immunosuppressive in some host populations, but not others. Consequently, its effects on other parasites would vary from one host population to the next. For instance, experimental infections by the tapeworm Schistocephalus solidus induce different kinds of immune responses in different populations of its host the threespine stickleback (Gasterosteus aculeatus). Some stickleback genotypes actively suppress immunity after tapeworm infection (by up-regulating immunosuppressive genes), whereas other populations initiate an aggressive immune phenotype that kills or limits tapeworm growth (e.g., more reactive oxygen production, and extensive fibrosis; Weber et al. 2017a, b, 2022; Fuess et al. 2021). Conversely, host immunity may induce the evolution of new immunosuppressive parasite variants; for instance, the omicron variant of SARS-CoV-2 gained enhanced inhibition of host MHC-I expression (Moriyama et al. 2023), whose frequency varied globally. This geographic mosaic of host (and parasite) traits has typically been overlooked in studies of among-parasite interactions, especially because lab-based experiments frequently focus on single host, or parasite, genotypes, to artificially reduce noise and complexity. Yet, that noise may itself be a feature of interest. Here, we present observational data to test our prediction that the helminth parasite Schistocephalus solidus might facilitate co-infection by other parasites, but that this effect on others would vary among host populations.
Study system
Like many parasites, S. solidus has a complex relationship with its host (the threespine stickleback), the host immune system, and other parasites. Research on stickleback from lakes in Germany revealed strong evidence for immune suppression and manipulation by S. solidus (Scharsack et al. 2007). During early stages of infection with S. solidus, stickleback increase granulocyte numbers (Scharsack et al. 2004). However, stickleback exposed to S. solidus that go on to develop infections demonstrate no differences in lymphocyte proliferation compared to control fish, while stickleback that are able to clear this infection have significant changes in lymphocyte proliferation (Scharsack et al. 2004). In British Columbia, stickleback from some lakes exhibit increased expression of anti-inflammatory and anti-fibrotic gene pathways, suggestive of parasite-induced immune suppression (Fuess et al. 2021). Yet, host populations clearly vary in their immune response to S. solidus, and their ability to limit or eliminate infections (Weber et al. 2017a, b, 2022; Fuess et al. 2021; Hund et al. 2022). Some populations are able to drastically limit cestode growth through a combination of inflammatory reactive oxygen species and fibrosis (Weber et al. 2022), and are frequently able to encase S. solidus in a fibrotic granuloma to eventually kill the parasite. These more resistant populations tend to exhibit more frequent peritoneal fibrosis, very low S. solidus prevalence, and smaller S. solidus when present (Weber et al. 2022; de Lisle and Bolnick 2020). Transcriptomic comparison of a resistant versus susceptible lake population found that the latter were more prone to infection-induced up-regulation of immune-suppressing gene pathways (Fuess et al. 2021). Based on this combination of evidence for (i) S. solidus-induced immune suppression, and (ii) among-population variation in stickleback immunity, we predicted that S. solidus may tend to facilitate co-infections by other parasite species, but that this effect would be strongest in susceptible/tolerant populations, and weak or reversed in more immunologically responsive hosts. Here, we present an observational (correlational) test of this prediction.
Methods
Collection
In June 2009, 60–100 stickleback were collected from each of 33 lakes on Vancouver Island in British Columbia, Canada. Fish were captured in unbaited 0.5-cm gauge wire minnow traps set for less than three hours along ~ 200 m of shoreline in 0.5–3-m-deep water. Immediate euthanization via MS222 was performed followed by preservation in 10% buffered formalin. Collection and animal handling were approved by the University of Texas IACUC (Protocol #07-032201), and a Scientific Fish Collection Permit from the Ministry of the Environment of British Columbia (NA07-32612). Collections occurred in the historical lands of the Kwakwaka'wakw First Nations. See Bolnick and Ballare 2020; Bolnick et al. 2020a; and Peng et al. 2021 for additional methods. The full dataset is available as a supplement to Bolnick et al. (2020a) (https://datadryad.org/stash/dataset/doi:10.5061/dryad.gmsbcc2j1). Code and data for the current analyses are archived on Dryad (data: https://doi.org/10.5061/dryad.ht76hdrhp; code: https://doi.org/10.5281/zenodo.5818496).
All fish were weighed and measured and sex was recorded via dissection. During dissections, we counted macroparasites (helminths, crustaceans, molluscs, and microsporidia) in each fish using a stereodissection microscope. Skin, fins, armor plates, buccal cavity, and gills were all examined for parasite prevalence. We then dissected the body cavity and organs (liver, swim bladder, gonads, and eyes) and opened the digestive tract to check for the presence of additional parasites. Parasites were identified to the lowest feasible taxonomic unit (typically genus). For abundant gill parasites, we counted parasites only on the right side. For each taxon, we calculated per-population infection prevalence (proportion of fish infected) and abundance (mean number of parasites per fish) following Bush et al. (1997), and confidence intervals of proportions following Newcombe (1998).
Analysis
We calculated the number of distinct parasite taxa (‘richness’) for each individual fish, excluding Schistocephalus. We calculated the mean per-fish parasite richness within each lake, separately for individuals infected by S. solidus, and for fish uninfected by S. solidus (for the former, we did not count S. solidus toward the total richness). We then used Poisson general linear models (GLMs) within each lake to estimate the magnitude and significance of within-lake differences in parasite richness between infected versus uninfected individuals. For each lake, we retained the log response ratio (LRR) from the GLM as a metric of the infection effect; positive values are consistent with facilitation and negative values consistent with inhibition. We acknowledge that these analyses are correlational inferences from observations made on wild stickleback, with all the concomitant potential for unknown confounding variables. Other biological processes could generate positive or negative associations between parasites. Therefore, strong inferences about mechanism and process (e.g., facilitation and inhibition) require future experimental manipulations.
To evaluate the overall effect of S. solidus infection on other parasites throughout our data set (e.g., across all lakes), we used a single Poisson GLM mixed effect model (using glmer in R). Each individual fish’s parasite richness (subtracting S. solidus, if present) was regressed against their S. solidus infection status, log standard length, and sex. The GLM included a random intercept effect of population, and interactions between each fixed effect term and population (e.g., random slopes). We considered a series of simpler models omitting the random slopes, the main fixed effects, or both. We compared nested models using AIC, and χ2 tests of significance, to determine the model that best fit the observed data.
Next, we tested whether populations with more abundant S. solidus infection (i.e., higher infection rates) proportions exhibit more facilitation-like trends compared to populations with rare S. solidus presence. Populations with higher S. solidus prevalence also tend to have larger S. solidus, and less fibrosis (Weber et al. 2022), suggesting that the host immune response is weaker or less effective. In contrast, populations with an aggressive fibrotic response and stronger gene expression changes following infection tend to have relatively few, and small, cestodes (Weber et al. 2022; Fuess et al. 2021; Lohman et al. 2017). We used linear regression to test whether lakes’ LRR (measuring facilitation/inhibition) varied as a function of the lakes’ S. solidus prevalence. To confirm that this test is robust to our statistical method, we additionally used a Poisson general linear model to test this hypothesis. As described above, we regressed individual sticklebacks’ parasite richness on their infection status (S. solidus present/absent) with log length as a covariate and lake as a random effect, but now with the addition of lake-wide S. solidus prevalence, and an interaction between prevalence and individual infection status. We also included lake mean S. solidus mass as a covariate. If ‘facilitation’ is stronger in lakes with more successful tapeworms, we expected a significant interaction effect indicating an amplifying effect of abundant (or, large) tapeworms on the tapeworm’s effect on other parasite richness.
To understand how the facilitation-like effect occurs, we then focused on each observed parasite taxon in turn. We tested whether the presence/absence of S. solidus affects the presence/absence of the focal parasite: we first considered their co-dependency on population and sex and fish mass and interactions among these variables, retaining residuals of a binomial GLM. We then tested whether each focal species’ residuals are correlated with the S. solidus residuals.
The preceding analyses were repeated using total parasite load instead of taxonomic richness. To account for variation in total parasite abundance among taxa, we first normalize the abundance of each parasite within each fish by dividing its count by the maximum observed count for that parasite. We then add up these normalized intensities across parasites and divide by richness to get a metric of infection intensity, which we subjected to the same analyses as parasite richness. We also repeated the preceding analyses of parasite richness using other focal parasite species besides S. solidus, to evaluate whether other parasites exhibit similar facilitation-like effects. For these additional focal species, we used the ten most common parasites (highest mean prevalence across the entire dataset) and used the same analytical approaches to test whether the presence/absence of the focal parasite is associated with higher richness of non-focal parasites (controlling for population, host sex, and host mass).
Results and discussion
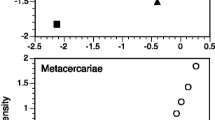
Per-fish parasite richness (excluding S. solidus) varied widely among stickleback populations, (Fig. 1A, see Bolnick et al. 2020a for further details). At the lowest end, the Campbell River Estuary population averaged a mere 0.45 parasite taxa per individual, compared to an average of 4.7 parasite taxa per individual in Grey Lake. As one would expect, comparing across lakes, the parasite richness of S. solidus-infected fish was strongly positively correlated with parasite richness of S. solidus-uninfected fish, paired by population (Fig. 1A, r = 0.76, P < 0.001, df = 19). Note that not all populations are included in Fig. 1A, because we necessarily exclude the populations where S. solidus was not present.
Effects of S. solidus infection on the diversity of co-infections. A Mean parasite richness (excluding S. solidus) of S. solidus-uninfected versus infected individuals, by population. Each point is a population, and error bars are ± 1 standard error confidence intervals along both axes (N = 20). Filled points represent statistically significant differences between infected versus uninfected fish within that lake; open circles are not significant (at alpha = 0.05, using a Poisson GLM), and thin black lines present lakes with fewer than three cestodes (omitted from subsequent analyses). A 1:1 diagonal line highlights equal parasite richness in both groups of fish in a lake. Points are colored in proportion to the effect size (Log Response Ratio) in the Poisson GLM, from blue (negative effect implying inhibition) to red (positive effect implying facilitation. B Averaging across all fish in lakes with cestode prevalence exceeding 1%, cestode presence increases the richness of other parasites. Here, we plot the mean and ± 1 standard error confidence intervals for uninfected and infected fish, using residuals calculated from a whole data set Poisson GLM regression on population, log length, and sex. The number of uninfected and infected fish is listed next to each point (N = 1802; N = 292). Inset is a photograph of a stickleback with the S. solidus cestode. C Effect of cestode prevalence, by population, on the S. solidus effect on other parasite richness. Effect sizes are measured as log response ratios, ln (mean parasite richness in cestode-infected fish/mean richness in cestode-free fish), which equals the effect slope in the Poisson general linear model. Lakes with fewer than three cestode-infected fish were dropped for this analysis (N = 15 populations used). Equivalent results are obtained if we instead use GLM Z scores. Points are shaded by as in A. A horizontal dashed line is added to emphasize the boundary between negative and positive effects (inhibition and facilitation, respectively), and a regression trendline is provided with statistical results in text at the bottom right
The diagonal line in Fig. 1A represents equal parasite richness for S. solidus-infected versus -uninfected individuals. Deviations above or below the line are consistent with, respectively, facilitation or inhibition of other parasites. Using a Poisson GLM within each lake, three populations exhibit a significant effect of S. solidus infection on other parasite richness (filled points in Fig. 1A). In all three lakes, individual fish with S. solidus present had significantly higher richness of other parasites, compared to S. solidus-uninfected fish from the same lake, consistent with our hypothesis of facilitation [Bob Lake log response ratio (LRR) = 0.54, P = 0.023; Gosling Lake LRR = 0.20, P = 0.043; and Merrill Lake LRR = 0.51, P = 0.033]. If we instead use t-tests, S. solidus significantly affects other parasite richness in five lakes (four lakes consistent with facilitation, one lake consistent with inhibition).
Across all populations on average, we find that S. solidus infection in individuals is associated with higher richness of other parasites. Several distinct statistical methods support this inference. First, the simplest approach is to contrast all infected versus all uninfected fish (excluding lakes with no observed S. solidus). On average for the surveyed metapopulation (excluding cestode-free lakes), S. solidus-infected stickleback carried 2.78 other parasite species, compared to 2.34 for uninfected individuals (t-test; t = 3.95, P < 0.0001), representing a 1.19-fold increase in parasite richness. Figure S1 plots the distribution of all observations rather than mean and standard error. This approach confounds among-lake variance and among-individual variance, so we next regressed parasite richness on population and fish size and sex, obtained residuals, and then tested whether these residuals differed between infected and uninfected fish, confirming the prior result (Fig. 1B, t-test of residuals: t = 2.50, P = 0.0129). Finally, we used a Poisson GLM with mixed effects to test whether, on average across the metacommunity, individual fish parasite richness depended on population (random intercept effect), log fish length, sex, S. solidus infection status (present/absent), and random intercept effects (population × length, population × sex, and population × S. solidus). AIC model selection favors a final model that retains a main effect of S. solidus on other parasite richness (Table 1, model 5), as well as effects of fish length, sex, population, and a length*population interaction. Likelihood ratio tests comparing this best model against one omitting S. solidus confirm that the effect is statistically significant (χ2 = 5.44, P = 0.0197).
Schistocephalus prevalence varied from 0% (24 populations, excluded for most analyses here) to as high as 74% (Merrill Lake, CI 57%-87%). The effect of S. solidus on other parasites tended to be negative or absent in populations with scarce S. solidus and increased toward a stronger positive effect in populations with abundant S. solidus (Fig. 1C; this analysis excludes lakes with fewer than three cestode-infected fish). Linear regression of the estimated S. solidus effect size (log response ratio from each lake’s Poisson GLM) on cestode prevalence revealed a significant positive relationship (slope = 0.88, s.e. = 0.26, P = 0.0056). An alternative statistical test (Poisson mixed model glm) supported the same inference, as there was a significant interaction between population prevalence and infection status, showing that the effect of S. solidus was stronger in lakes where the tapeworms were more prevalent (LRR = 0.67, s.e. = 0.29, P = 0.0193) and larger (marginally non-significant effect, LRR = 1.0, s.e. = 0.55, P = 0.068). Prior studies have confirmed that in some of these high-infection lakes, the resident stickleback mount a relatively weak immune response to S. solidus (Weber et al. 2017a, 2022) and even exhibit up-regulation of immunosuppressive gene pathways (Fuess et al. 2021), compared to more resistant populations with low prevalence that initiate stronger inflammatory and fibrotic immune response. The positive relationship in Fig. 1C is thus consistent with the hypothesis that successful cestode populations are able to suppress host immune functions (as shown by Scharsack et al. 2007; Fuess et al. 2021), thereby facilitating co-infection by other parasites. In contrast, in host populations that mount effective immune responses (e.g., Roselle Lake), the cestode is rare and when present tends to inhibit other parasites (Weber et al. 2022). This inhibition-like trend might represent collateral damage mediated via the host’s more active immune response.
Of course, readers must bear in mind that these trends are correlational and might arise from other causes. Our hypothesized explanation could be tested further by assaying the strength of stickleback immune responses to S. solidus, from high- and low-prevalence lakes, in lab infection assays. Importantly, the observed geographic variation in S. solidus’s effects on other parasites is more likely to be due to host evolutionary divergence, than to S. solidus evolution. This is because the stickleback hosts are confined to lakes and exhibit substantial among-population genetic divergence. In contrast, S. solidus is dispersed by its terminal host (piscivorous birds such as loons) and exhibits an order of magnitude less population genetic structure (Shim et al. 2021).
We next considered how individual parasite taxa were affected by S. solidus. Using binomial GLMs, we obtained residuals from regression of S. solidus infection on lake, fish size, and sex, and then also obtained residuals for each other parasite taxon. We then used regression to test for an association between S. solidus residuals, and each other parasite. Residual S. solidus infection was positively correlated with residual infection risk from Neoechinorhyncus sp. (t = 4.65, P < 0.0001), blackspot (t = 1.96, P = 0.0508), Cystidicola sp. (t = 2.44, P = 0.0149), Anisakis sp. (t = 1.922, P = 0.0549), Glugea anomalis (t = 2.87, P = 0.0042), and an unidentified nematode (t = 3.26, P = 0.0012). These positive trends are in a direction that is consistent with our inference of facilitation, and collectively would explain the increase in parasite richness. Notably, none of the gill-infesting parasites (Unionidae, Thersitina, and Ergasilus) were affected, consistent with expectations for ectoparasites that are typically less sensitive to host immune status. These exceptions to the positive trend lend further support to our proposed explanation for the observed patterns.
We evaluated whether other metrics (of parasitism or of S. solidus infection) showed similar facilitation-like effects. Using Poisson GLMs (with lake, log fish length, and sex as covariates), we found no significant effect of S. solidus intensity either as a main effect (P = 0.688) or interaction with population (P = 0.986). The average cestode mass also had no main effect or interaction with population (P = 0.408, interaction P = 0.562). Cestode presence/absence had no significant effect on total parasite load, though the trends were in the same overall direction as parasite richness showed.
We framed our study around S. solidus because this parasite has prior evidence of immunosuppressive effects in laboratory studies, and so is likely to cause facilitation. Having tested this a priori hypothesis, we subsequently chose to conduct a posteriori tests of whether other parasites have similar effects on parasite richness. Repeating the above analyses, we found several instances in which a different focal parasite had positive effects on non-focal parasite richness (Table 2). An Acanthocephalan (Neoechinorhyncus sp.), a digenean flatworm (Crepidostomum sp.), and a trematode (Diplostomum sp.) all showed at least as strong a facilitation-like effect as S. solidus (all P < 0.01). Illustrative examples are provided in Supplementary Figures S2-S4). Surprisingly, two external parasites also showed similar trends (Thersitina, P < 0.001, and Dermocystidium, P = 0.021). However, none of these parasites exhibit the positive relationship between putative prevalence and facilitation effect, that was seen in S. solidus (Fig. 1C, Table 2). Several other parasites showed no sign of facilitation, including two external parasites (Unionidae, Ergasilus), and other helminths (Bunodera [Supplementary Figure S4], Eustrongyloides, and Proteocephalus). The parasite species showing positive effects on parasite richness are very weakly correlated or un-correlated with S. solidus (with the exception of Neoechinorhyncus), so their effects are independent. Overall, the tendency for infection by multiple focal parasites, to coincide with greater infection by other parasites, does suggest a more general (not S. solidus-specific) process. One such process could entail inherent variation in stickleback immunocompetence (e.g., due to genotype, or past foraging success, breeding status, etc.). Generally susceptible fish would be multiply infected, and each of those parasites would exhibit facilitation-like effects on other parasites. However, the counterargument is that only S. solidus has prior experimental evidence of immunosuppression, and only S. solidus exhibits a positive correlation between its population prevalence, and its facilitation-like effect on other parasites.
To conclude, we have demonstrated that in a large sample of stickleback from a genetically and ecologically heterogeneous metapopulation, S. solidus infections tend to coincide with a higher richness of other parasites co-infecting the same individual fish. Schistocephalus solidus is known to manipulate its host’s phenotype, including gene expression (Fuess et al. 2021; Weber et al. 2022), immune traits (Weber et al. 2017a, 2022), and behavior (Grecias et al. 2020; Berger and Aubin-Horth 2020). This immune suppression is a likely explanation for the facilitation observed here. However, we also know that host populations differ in their ability to prevent, and limit, infections via heritable differences in their gene expression response to infection and immune phenotypes (Lohman et al 2017; Fuess et al 2021; Weber et al. 2017a). Since host evolution has led to differences in immune responses to infection, across the stickleback metapopulation, it is likely that S. solidus’s facilitation effect may be negated or reversed in more immunologically reactive host populations. Consistent with this notion, we find that facilitation-like effects are strongest in populations with successful S. solidus (high prevalence), and in populations where S. solidus is rare (Fig. 1B). These results are consistent with our hypothesis that the outcome of indirect interactions between parasites, mediated via evolving host traits, will vary across a landscape due to microevolution of host immunity. In effect, the geographic mosaic of co-evolution (between fish and cestode) could lead to another geographic mosaic of facilitation structuring the parasite metacommunity. This observation may provide a path toward a mechanistic explanation for the recently reported structure of the stickleback parasite metacommunity (Bolnick et al. 2020b). That study showed that a number of combinations of parasite species tended to co-occur or were negatively correlated. But puzzlingly, the species co-occurrence network was inconsistent from one lake to the next. Our present results suggest that host immune evolution may substantially modify indirect interactions among parasites.
References
Adegnika AA, Kremsner PG (2012) Epidemiology of malaria and helminth interaction: a review from 2001 to 2011. Curr Opin HIV AIDS 7:221–224
Balmer O et al (2009) Intraspecific competition between co-infecting parasite strains enhances host survival in African trypanosomes. Ecology 90:3367–3378
Berger CS, Aubin-Horth N (2020) The secretome of a parasite alters its host’s behaviour but does not recapitulate the behavioural response to infection. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2020.0412
Bolnick DI, Ballare KM (2020) Resource diversity promotes among-individual diet variation, but not genomic diversity, in lake stickleback. Ecol Lett 23:495–505
Bolnick DI et al (2020a) Host patch traits have scale-dependent effects on diversity in a stickleback parasite metacommunity. Ecography (cop) 43:990–1002
Bolnick DI et al (2020b) Scale-dependent effects of host patch traits on species composition in a stickleback parasite metacommunity. Ecology. https://doi.org/10.1002/ecy.3181
Bush AO et al (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Colwell DA, Wescott RB (1973) Prolongation of egg production of Nippostrongylus brasiliensis in mice concurrently infected with Nematospiroides dubius. J Parasitol 59:1–216
de Lisle SP, Bolnick DI (2020) Male and female reproductive fitness costs of an immune response in natural populations. Evolution 75:2509–2523
Ezenwa VO et al (2010) Hidden consequences of living in a wormy world: nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. Am Nat 176:613–624
Fuess LE et al (2021) Between-population differences in constitutive and infection-induced gene expression in threespine stickleback. Mol Ecol 30:6791–6805
Grecias L et al (2020) Host behaviour alteration by its parasite: from brain gene expression to functional test. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2020.2252
Griffiths EC et al (2011) The nature and consequences of co-infection in humans. J Infect 63:200–206
Hammarlund SP et al (2019) A shared limiting resource leads to competitive exclusion in a cross-feeding system. Environ Microbiol 21:759–771
Hellard E et al (2015) Parasite-parasite interactions in the wild: how to detect them? Trends Parasitol 31:640–652
Hund AK et al (2022) Population-level variation in parasite resistance due to differences in immune initiation and rate of response. Evol Lett 6:162–177
Jolles AE et al (2008) Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology 89:2239–2250
Lohman BK et al (2017) Gene expression contributes to the recent evolution of host resistance in a model host parasite system. Front Immunol 8:1071
Moriyama M, MonteiroIwasaki VSA, Yale SARS-CoV-2 Genomic Surveillance Initiative (2023) Enhanced inhibition of MHC-I expression by SARS-CoV-2 Omicron subvariants. Proc Natl Acad Sci USA 120:e2221652120
Newcombe RG (1998) Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17:857–872
Ngwenya BZ (1982) Enhanced resistance to Plasmodium berghei in mice previously infected with Trichinella spiralis. Parasite Immunol 4:197–207
Peng F et al (2021) What evolutionary processes maintain MHC IIβ diversity within and among populations of stickleback? Mol Ecol 30:1659–1671
Råberg L et al (2006) The role of immune-mediated apparent competition in genetically diverse malaria infections. Am Nat 168:41–53
Scharsack JP et al (2004) Modulation of granulocyte responses in three-spined sticklebacks Gasterosteus aculeatus infected with the tapeworm Schistocephalus solidus. Dis Aquat Organ 59:141–150
Scharsack JP et al (2007) Who is in control of the stickleback immune system: Interactions between Schistocephalus solidus and its specific vertebrate host. Proc R Soc B Biol Sci 274:3151–3158
Shim KC et al (2021) Population genomics of a threespine stickleback tapeworm in Vancouver Island. BioRxiv Preprint. https://doi.org/10.1101/2022.05.15.491937
Telfer S et al (2010) Species interactions in a parasite community drive infection risk in a wildlife population. Science 330:243–246
Thompson JN (2004) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Thumbi SM et al (2014) Parasite co-infections and their impact on survival of indigenous cattle. PLoS ONE 9:e376324
Turner CMR et al (1996) Inhibition of growth of Trypanosoma brucei parasites in chronic infection. Parasitol Res 82:61–66
Weber JN, Steinel NC et al (2017a) Recent evolution of extreme cestode growth suppression by a vertebrate host. Proc Natl Acad Sci USA 114:6575–6580
Weber JN, Kalbe M et al (2017b) Resist globally, infect locally: a transcontinental test of adaptation by stickleback and their tapeworm parasite. Am Nat 189:43–57
Weber JN et al (2022) Adaptive evolution of a protective but costly immune response to a helminth parasite. Science 377:1206–1211
Acknowledgements
The authors would like to thank C. Harrison, T. Rodbumrung, and T. Ingram, for assistance with field work to collect samples. J. Day and K. Ballare assisted with parasite data collection.
Funding
Sample collection was supported by the David and Lucille Packard Foundation. Data acquisition was funded by a Howard Hughes Medical Institute Early Career Scientist award to DIB. Analysis and writing were supported by the National Institutes of Health (1R01AI123659-01A1) to DIB, and by a Gordon and Betty Moore Foundation Aquatic Symbiosis grant (DIB).
Author information
Authors and Affiliations
Contributions
DIB originally formulated the idea, DIB conducted fieldwork, MLR and DIB performed analyses, and MLR and DIB wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
Collection and animal handling were approved by the University of Texas IACUC (Protocol #07-032201), and a Scientific Fish Collection Permit from the Ministry of the Environment of British Columbia (NA07-32612).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
For the research herein, data are already published and publicly available, as noted in the Materials and methods: The full dataset is available as a supplement to (Bolnick et al. 2020a) (https://datadryad.org/stash/dataset/doi:10.5061/dryad.gmsbcc2j1). Data for the current analyses are archived on Dryad (data: https://doi.org/10.5061/dryad.ht76hdrhp; code: https://doi.org/10.5281/zenodo.5818496).
Code availability
Code for the current analyses are archived on Dryad (data: https://doi.org/10.5061/dryad.ht76hdrhp; code: https://doi.org/10.5281/zenodo.5818496).
Additional information
Communicated by Tara Merrill and Jason Hoverman.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodgers, M.L., Bolnick, D.I. Opening a can of worms: a test of the co-infection facilitation hypothesis. Oecologia 204, 317–325 (2024). https://doi.org/10.1007/s00442-023-05409-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-023-05409-7