Abstract
The persistence of wildlife populations is under threat as a consequence of human activities, which are degrading natural ecosystems. Commercial forestry is the greatest threat to biodiversity in boreal forests. Forestry practices have degraded most available habitat, threatening the persistence of natural populations. Understanding population responses is, therefore, critical for their conservation. Population viability analyses are effective tools to predict population persistence under forestry management. However, quantifying the mechanisms driving population responses is complex as population dynamics vary temporally and spatially. Metapopulation dynamics are governed by local dynamics and spatial factors, potentially mediating the impacts of forestry e.g., through dispersal. Here, we performed a seasonal, spatially explicit population viability analysis, using long-term data from a group-living territorial bird (Siberian jay, Perisoreus infaustus). We quantified the effects of forest management on metapopulation dynamics, via forest type-specific demography and spatially explicit dispersal, and how forestry impacted the stability of metapopulation dynamics. Forestry reduced metapopulation growth and stability, through negative effects on reproduction and survival. Territories in higher quality natural forest contributed more to metapopulation dynamics than managed forests, largely through demographic processes rather than dispersal. Metapopulation dynamics in managed forest were also less resilient to disturbances and consequently, may be more vulnerable to environmental change. Seasonal differences in source-sink dynamics observed in managed forest, but not natural forests, were caused by associated seasonal differences in dispersal. As shown here, capturing seasonal source-sink dynamics allows us to predict population persistence under human disturbance and to provide targeted conservation recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wildlife populations are facing a plethora of threats as a consequence of human activities, which are altering and degrading natural ecosystems (Tilman et al. 2017). Commercial forestry alters forest composition, reduces habitat quality (i.e., habitat degradation), and increases forest fragmentation (Imbeau et al. 2001), with potentially severe impacts on the persistence of natural populations (Noble and Dirzo 1997). Accordingly, forestry management is considered the greatest threat to biodiversity in boreal forests, the largest terrestrial ecosystem. Understanding how populations respond to the effects of commercial forestry, and the implications for their persistence, is therefore imperative for their conservation (Rahmstorf and Coumou 2011; Thompson et al. 2013).
Pulliam (1988) and others (Howe et al. 1991; Dias 1996) used the ‘source-sink’ concept to quantify the demographic consequences of varying habitat quality. Following this concept, several approaches to modelling source-sink dynamics have been developed (Thomas and Kunin 1999; Figueira and Crowder 2006). Population viability analyses (PVAs) provide important conservation tools to predict population persistence under environmental change (Boyce 1992; Akçakaya 2000; Hanski and Gaggiotti 2004). Spatial PVAs have been used to assess how habitat degradation and fragmentation affects population persistence and extinction risk (Doak 1989; Lamberson et al. 1992; Akçakaya and Atwood 1997). Spatial PVAs therefore represent a potentially effective conservation tool, to identify source sites that support less productive sites, although source versus sink characteristics of a given patch can fluctuate in time (Akçakaya et al. 1995; Ozgul et al. 2009).
Temporal environmental stochasticity can lead to fluctuations in population sizes and thus a populations extinction risk (Lande 1993; Fagan et al. 2001). However, seasonality (i.e., a form temporal variability) is not often explicitly accounted for in PVA frameworks, even though most natural populations live in seasonal environments, leading to cyclical changes in vital rates (Fretwell 1972; Norris and Marra 2007). Seasonality may have important consequences for population dynamics (Kot and Schaffer 1984; Stenseth et al. 2003), especially in spatially structured populations, which are driven by stochastic local dynamics and spatial factors (e.g., dispersal), which can both vary seasonally (Paniw et al. 2019, e.g., Behr et al. 2020).
Prospective and retrospective perturbation analyses are commonly used to quantify the relative importance of vital rates for a population’s fitness (Demetrius 1969; Goodman 1971). Later developments extended the application of perturbation analysis to spatially structured populations (Hunter and Caswell 2005), allowing us to quantify the relative importance of local populations for metapopulation dynamics (Ozgul et al. 2009). Such perturbation analyses are similar to the assessment of ‘patch values’, i.e., the contribution of a local population to metapopulation dynamics (Hanski and Ovaskainen 2000). However, perturbation analyses only focus on the largest eigenvalue (i.e., the asymptotic population growth rate), whereas the ratio between the two largest positive eigenvalues or ‘damping ratio’ (Koons et al. 2005) can provide information on a populations resilience to changes in underlying rates. Although damping ratios (and similar concepts) have been used in the context of metapopulation occupancy models (e.g., Day and Possingham 1995; Bode et al. 2008; Shima et al. 2010), they have not applied to spatially structured population projection matrices.
Here, we developed a metapopulation model, combining periodic (Caswell and Trevisan 1994) and vec-permutation matrix approaches (Hunter and Caswell 2005). We implemented environmental fluctuations in metapopulation dynamics via seasonal vital rates. We applied this approach to a population of Siberian jays, Perisoreus infaustus, a social bird species, where groups occupy year-round stable territories in boreal forests (Ekman and Griesser 2016). In boreal forests, birds constitute the majority of terrestrial vertebrate species, making this a representative group to focus conservation efforts (Niemi et al. 1998). Territories can be considered as ‘patches’ within a metapopulation since they tend to maintain a stable location among years. They also exhibit asynchrony in local demography and partial dispersal among territories, following the definition of a metapopulation (Fryxell 2001). The study population inhabits two distinct areas within a heterogeneous landscape; one is located in pristine (‘natural’, i.e., not managed for the previous 200 years) forests while the other is located in managed forest consisting of a matrix of heavily managed patches intermixed with few unmanaged patches. Commercially managed forest is generally less dense than natural forest, as the understory is removed several times over a growing cycle. This increases adult predation risk by visually hunting hawks and owls, and also nest predation risk by other corvids (Griesser et al. 2006, 2007, 2017). Consequently, forestry management increases mortality and reproductive failure in Siberian jays (Griesser et al. 2007; Layton-Matthews et al. 2018). By modelling the dynamics of a Siberian jay metapopulation (70 territories), located in natural and managed forest, we quantified the effects of forestry on seasonal metapopulation dynamics and metapopulation stability.
Materials and methods
Study system
Data were collected in an individually colour-ringed population of Siberian jays, in northern Sweden (65°40' N, 19°10' E). The study site, near Arvidsjaur, is located in boreal forests dominated by Scots pine (Pinus silvesteris) and Norway spruce (Picea abies). The site is separated into two areas (south and north) with differing forest management. Forests in the southern area are managed, which involves repeated thinning, harvesting and replanting in 80–120 year-long cycles, resulting in more open, even-aged forest (Griesser et al. 2007; Griesser and Lagerberg 2012). Forests in the northern area have not been managed in the last 200 years and are structurally more diverse and unevenly aged. Hereon, natural forest territories refer to the northern area consisting of predominantly pristine forest (i.e., not managed for the past 200 years). Territories in managed forest included those that were subject to forest management during the study period, where forest management refers to both thinning and clearcutting practices (Griesser et al. 2007).
We used data collected between 2000 and 2014, from 70 territories in natural (n = 28) and managed (n = 42) forest (see Appendix S1, Table S1 for annual sample sizes). Siberian jays live in family groups generally including a monogamous breeding pair, and 1 − 5 retained offspring and unrelated non-breeders (average group size = 3.5, range = 1 – 7, based on field observations). Retained offspring remain with their parents for up to four years and force their subordinate siblings to disperse from their natal territory soon after fledging. Dominance is reflected in the hatching order, where first hatched individuals have a competitive advantage (Ekman and Griesser 2016). Parents provide retained offspring with nepotistic care, resulting in higher lifetime fitness for individuals delaying dispersal (Ekman et al. 1999; Ekman and Griesser 2002). Breeding occurs in April–May, and subordinate juveniles (i.e., early dispersing juveniles) leave their natal territory during their first summer. All territories were visited repeatedly in March, before the breeding season, and in September, after juveniles have dispersed and settled in another group. Social information was used to determine the rank of all group members (Griesser et al. 2015). We determined the territory core based on nest locations collected from 2000 to 2004 and 2011 to 2013, since Siberian jays appear to focus their time in the area core to the breeding site and territories locations are generally stable across years (Nystrand et al. 2010).
Local population dynamics
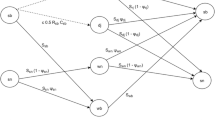
We used previously estimated seasonal vital rates to describe demography in natural and managed forests. Vital rates were estimated separately for juveniles (new recruits observed in winter), non-breeding and breeding individuals. Life-history stages were also on a seasonal-basis: divided into a summer (March–August) and winter (September–February) phase (Appendix S2, Layton-Matthews et al. 2018). The summer season encompasses the breeding season and main period of juvenile dispersal, while winter is outside the breeding season and dispersal is more limited during this period. Since breeding pairs represent dominant individuals in a social group and provide extended nepotistic care to their offspring, non-breeder/breeder stages apply throughout the year. Transitioning to a breeder only occurs when a breeding position becomes vacant. Consequently, vital rates were specific to the following life-history stages; dispersed juvenile (dj, i.e., individuals that disperse from the natal territory soon after fledging), retained juvenile (rj, i.e., retained offspring that remain in their natal territory), summer (sn) and winter (wn) non-breeder and summer (sb) and winter (wb) breeder (Fig. 1).
Siberian jay life cycle with life-history stages; retained juvenile (rj), dispersed juvenile (dj), summer non-breeder (sn), winter non-breeder (wn), summer breeder (sb) and winter breeder (wb). The vertical dashed line separates the summer and winter seasons. Sx is the probability of an individual in stage x surviving and Ψx is the probability of an individual in stage x transitioning to a breeder stage at the next census. Rsb is the probability of a breeding pair producing an offspring and Csb is the number of offspring per breeding pair that remains in their natal territory as a retained juvenile. Dispersed juvenile recruitment is retained juvenile recruitment multiplied by c (Methods)
Survival (Sdj,rj,sn,wn,sb,wb), recruitment (Rsb, Csb) and transition rates (i.e., transition probability to a breeding stage conditional on survival, Ψdj,rj,sn,wn) were based on the life cycle in Fig. 1. Recruitment described the number of retained juveniles per (summer) breeder in September and was based on two parameters: recruitment rate (Rsb, proportion of summer breeders recruiting a juvenile into the retained juvenile stage) and number recruited (Csb, number of retained juveniles recruited per summer breeder). Recruitment of retained juveniles was equal to Rsb × Csb × 0.5, to account for female-based reproduction. Recruitment of dispersed juveniles was further multiplied by a correction factor (c) to account for 20% dispersal mortality (Griesser et al. 2014) and the proportion of juveniles dispersing (Pdj, following paragraph).
Following Layton-Matthews et al. (2018), we parameterised two periodic, stage-structured, population matrices (Ksummer, Kwinter), which describe demographic processes from summer to winter and from winter to summer, for natural and managed forest (Caswell and Trevisan 1994; Caswell 2001). The winter matrix (right, Kwinter) projected the population from the two summer stages (sn and sb) onto four winter stages (rj, dj, wn and wb). The summer matrix (left, Ksummer) projected the four winter stages (rj, dj, wn and wb) onto the two summer stages (sn and sb).

The product of these periodic matrices, A, projects the population through an entire annual cycle.
where the population vector, n(t), gives the density of each life-history stage, in the season at which the projection started.
Metapopulation modelling
Dispersal
Developing a spatial model requires knowledge of dispersal processes. Dispersal events occurred when an individual was recorded in a different territory at season t + 1 from season t. Data on dispersal events were based on mark-recapture data (seasonal observations of individuals in a given territory) and additional radio-tagging data of dispersed juveniles (to determine the location of juveniles in season t). In Siberian jays, dispersal is strongly life history stage-specific and varies between natural and managed forests (Griesser et al. 2014). We estimated dispersal rates (Pd, i.e., proportion of individuals dispersing) and tested whether rates were life-history stage and forest-specific, by fitting the data as a binomial distribution and used AICc-based model selection to select the most parsimonious model (Table S1, Appendix S3). We modelled dispersal distance (d) as the distance between two territory cores. Data were fitted with five common density functions (i.e., dispersal kernels functions, Clark et al. 1999; Hastings et al. 2005; Van Houtan et al. 2007). We identified the best-fitting kernel and differences between life-history stage and forest type using AICc-based model selection (Table S2, Appendix S3).
Spatial population model construction
Equation (1) can also be used to describe a spatial matrix model, where the population vector (n) includes the densities of life-history stage s in each local population (referred to as ‘patch’, p). This spatial (metapopulation) projection matrix, AMP, incorporates patch and stage-specific demographic and dispersal rates. Spatial matrix models require three components: the state of the metapopulation (state- and patch-specific densities), the demographic processes in each patch, dispersal of individuals among patches (Hunter and Caswell 2005). In this case, AMP, projects the metapopulation from winter to summer and vice versa, based on local population matrices.
Here, the metapopulation state or population vector, ni,j(t), is described by the density at stage i and patch j, where the sub-vectors give the stage distribution within each patch.
We modelled demography without dispersal using the demographic projection matrix B, based on the seasonal population matrices (Ksummer, Kwinter). For each season, B is a block diagonal matrix, ordered by patches. The ith block of Kwinter,i is a 2 × 4 matrix and Ksummer,i is a 4 × 2 matrix.
We modelled dispersal (based on estimated dispersal rates, Pd) using the dispersal projection matrix M, for each season (winter and summer). M is a block diagonal matrix, ordered by (winter or summer) stages, where the ith block Li is a p × p matrix describing dispersal rates between patches,
where s is the number of stages. The sub-diagonal of Mwinter was used to re-distribute retained juveniles to the dispersed juvenile stage, based on the juvenile dispersal rate (Pdj).
With this approach, we assumed that demography and dispersal occurred sequentially, with demography occurring first. To conserve the block diagonal forms of B (ordered by patches) and M (ordered by stages), we used a vec-permutation matrix (Ps,p) to convert a population vector organised by patches \(\left( {\left| {n_{1} \vdots n_{p} } \right|} \right)\) to one organised by stages \(\left( {\left| {n_{1} \vdots n_{s} } \right|} \right)\) and vice versa (see Hunter and Caswell 2005 for details). This metapopulation projection matrix (AMP) can be written as
where \(|{n}_{1}\vdots {n}_{p}{|}_{t}\) is the initial metapopulation vector, ordered by patch at season t, and \({n}_{p}\) is the population vector for the pth patch ordered by stage. Psummer and Pwinter convert the population vector from a one ordered by patches to one ordered by stages during summer and winter respectively and vice versa for the corresponding transposes (PseasonT).
Forest model
We first constructed a spatial population model based on two local populations (i.e. p = 2), one occupying natural forest and the other in managed forest (hereon referred to as the ‘Forest model’). Bwinter is a 4 × 8 matrix describing transitions from two winter stages to four summer stages, for each patch (Kp = 2). Bsummer is an 8 × 4 matrix (four winter stages to two summer stages). Mwinter is an 8 × 8 matrix where the ith diagonal block Li is a 2 × 2 matrix for each of the four winter stages. Msummer is a 4 × 4 matrix for each of the two summer stages.
Territory model
Second, we constructed a spatial population model at the territory level (i.e. 70 territories/patches) with spatially explicit dispersal, hereon referred to as the ‘Territory model’. Consequently, for the demographic matrix B, Kp = 70 and the dimensions of Li are 70 × 70 for stage i. To implement spatially explicit dispersal, individuals were forced to disperse within AMP at each time step (season), based on distance-dependent dispersal assuming a closed system. Specifically, we quantified the proportion of individuals dispersing into each distance class, based on distance- and stage-specific dispersal distances. We used a lognormal model (Hanski and Thomas 1994; Tischendorf and Fahrig 2000) with Dij as the response variable describing the number of individuals in patch i moving to patch j.
where dij is the midpoint of each distance class and μx and σx correspond to the mean and variance in dispersal distances, respectively (Moilanen 2004).
Analyses
Metapopulation growth
We calculated asymptotic local population growth rates (natural and manged forest) and the metapopulation growth rate, λMP, (i.e., the asymptotic growth rate across all territories). We estimated confidence intervals for λMP, using a life-table simulation analysis (Wisdom et al. 2000) by simulating 10,000 replicate projection matrices of AMP, using demographic and dispersal rates sampled from their statistical distributions (Table 1).
Parameter-level perturbation analysis of the Forest model
We applied a lower-level prospective perturbation analysis to the Forest model (Table 2), to determine the relative influence of forest-specific demographic and dispersal rates on λMP. Elasticities were determined analytically using the chain rule for periodic matrices (Caswell and Trevisan 1994; Lesnoff et al. 2003). We performed a fixed, one-way life-table response experiment (LTRE), a common retrospective perturbation analysis (Caswell 1989), to quantify the relative contributions of demographic and dispersal rates to the difference in λMP between natural and managed forest. In this analysis, the contribution of vital rate x to the difference in λMP was the product of the difference in x between natural and managed forest, and the sensitivity of λMP to x (calculated for the natural forest matrix).
Territory-level elasticity analysis of the Territory model
Like the Forest model, we calculated elasticities of λMP to lower-level parameters in each patch (i.e., territory). However, here we summed the elasticities to demography and dispersal parameters (see allocation in Table 1), to give the relative influence of demographic and dispersal in each patch on metapopulation growth, for the summer and winter projections (Appendix S4).
We also calculated the connectivity of each patch, to determine the relationship between a patch’s connectivity to neighbouring patches and its influence on metapopulation growth (λMP). Following Moilanen (2004), a patch’s connectivity, Ci, was calculated as \(C_{i} = \sum\nolimits_{j \ne i} {O_{j} \left( t \right)D\left( {d_{ij} ,\alpha } \right)A_{i} }\). Connectivity of focal patch i was the sum over all neighbouring patches j of the product of the occupancy statuses, Oj(t) of patch j (where 0 = absent and 1 = present), and the effective distance (Dij) between the focal and neighbouring patch, weighted by the number of individuals per patch (Ai, i.e., proxy for patch size). The function (Dij) defines the distribution of dispersal distances, where di,j is the distance between patches i and j and α defines the distributions of dispersal distances, where 1/α is the average dispersal distance (Moilanen 2004).
Damping ratios
To study the importance of dispersal processes for metapopulation stability, we performed a ‘trait-level analysis’ (Coste et al. 2017) on the metapopulation projection matrix (AMP) of the Territory model. Trait-level analysis can be used to determine the importance of a given life cycle component (e.g., breeding status, stage or spatial location) and, thus, of the underlying process (e.g., dispersal for spatial location). This method measures the relative importance of a category in a projection matrix (here, spatial location) by ‘folding’ the matrix over that category (see Coste et al. 2017). Using this approach, we calculated the damping ratio (ρ), i.e., the ratio of the dominant eigenvalue (λ1) to the second largest (sub-dominant) eigenvalue (λ2) (Caswell 2001). In this context, the damping ratio is a measure of the resilience of metapopulation growth to changes in dispersal and/or demography, where the lower the damping ratio, the more resilient the population is (see Appendix S5 for details on the methodological approach and a simplified example). We compared the ρ of the full metapopulation matrix, structured by (spatial) location and stage (AMP), with the same matrix ‘folded’ over location (i.e., structured by stage only, \({\bf {A}}_{\mathrm{MP}}^{\mathrm{fold}}\)), to determine the influence of dispersal on metapopulation stability. We also performed the same analysis for natural and managed forests separately (i.e., to test whether the importance of spatial structure differed between forests).
Management scenario
Using the territory model, we calculated λMP for a scenario where 25% of natural forest territories were removed from the metapopulation matrix, i.e., an expansion in forestry management.
Results
Local population dynamics
Previously estimated vital rates (from Layton-Matthews et al. 2018) reflected lower reproductive and survival rates in managed forest. However, stage-transition rates for juveniles and non-breeders to the breeder stage were higher in managed forest than in natural forest (Appendix S2). In addition, we modelled dispersal probabilities and dispersal distances. The best model of dispersal probabilities (Pd) included additive effects of forest type and life-history stage (Appendix S3, Table S1). Juvenile dispersal rate (Pdj) was significantly higher than non-breeders and breeders. Dispersal rates were higher in managed forest than natural forest (Appendix S3, Fig. S1). Dispersal distances (d) were best approximated with a lognormal dispersal kernel and differed between life-history stages since juveniles dispersed further than post-juvenile life-history stages (Appendix S3, Table S2).
Local population growth rates were 1.00 (CIs: 0.97, 1.03) in natural forest and 0.96 (0.93, 0.98) in managed forest, although CIs overlapped, reflecting lower survival and reproductive rates in managed forest (Table 3). Lower survival and recruitment rates in managed forest (and thus reduced λ) were, in part, counteracted by higher transition probabilities of juveniles and non-breeders to breeder status (Appendix S2).
Metapopulation dynamics
The asymptotic metapopulation growth rate (λMP, i.e., average λ across all territories) for the Territory model (i.e., with 70 territories and distance-dependent dispersal) was 0.98 (0.96, 1.00), indicating a stationary metapopulation (neither increasing nor decreasing in size) (Table 3). However, λMP was reduced to 0.97 (0.95, 0.99) when 25% of natural forest territories (7 territories) were removed from the metapopulation.
Metapopulation growth was more sensitive to demography in natural forest than managed forest. (Fig. 2a), but it was more sensitive to dispersal in managed forest than natural forest. Overall, demography had a greater influence on λMP than dispersal (Fig. 2a). The metapopulation growth rate was most sensitive to breeder survival (Ssb, Swb), followed by juvenile survival (Sdj, Srj) and recruitment parameters (Rsb, Csb) (Fig. 3a). Lower breeding survival and recruitment (Rsb) rates in managed forest were largely responsible for the difference in the contributions of natural and managed forest territories to λMP (Fig. 3b). However, higher transition rates to breeder status from juvenile and older stages in managed forest increased the metapopulation growth rate in managed forest (Fig. 3b).
Parameter-level perturbation analysis of the Forest model: a Elasticities of λMP to demographic rates; recruitment rate (Rsb), number of recruits (Csb), life history stage-specific survival (Sdj,rj,sn,wn,sb,wb) and transition probability to breeder (Ψrj,dj,sn,wn) dispersal rates (Pddj,sn,wn,sb,wb). Elasticities were calculated using the forest model (i.e., with spatially implicit dispersal), where each patch corresponds to a forest type: managed (grey) and natural (black). b Contributions of vital rates to the observed differences in λMP between natural and managed forest
Patch-level elasticity analysis of the Territory model: points represent the spatial location of a patch in natural (northern) and managed forest (southern), for a given latitude (x-coordinate) and longitude (y-coordinate). Patch colour corresponds to the elasticity of λMP to winter to summer demography (Bsummer, a), summer to winter demography (Bwinter, b), summer dispersal (Msummer, c) and winter dispersal (Mwinter, d), based on the territory model (i.e., spatially explicit dispersal, where one patch = one territory). Patch size corresponds to its connectivity
Source-sink dynamics
Based on elasticity analysis of the Territory model, the metapopulation growth rate was more sensitive to patch demography in natural than managed forest (Fig. 3). The elasticities of λMP to demography, summed across all territories in natural forest was 0.031, while it was 0.003 in managed forest territories. Elasticities to demography were similar across seasons and therefore only one estimate is shown. There was a positive relationship between patch connectivity and the elasticity of λMP to demography in natural forest (Fig. 3a, b). Between forest types, seasonal differences in demography (i.e., reproduction in summer and lower mortality in winter, Appendix S2) had little effect on the influence of patches on metapopulation growth (Fig. 3).
Metapopulation growth was more sensitive to dispersal in managed forest patches (Elas(Mx) = 0.020) than in natural forest patches (0.006), and this was consistent across seasons (x). Patch connectivity had no influence on the sensitivity of λMP to dispersal, although there was a large variation in the influence of dispersal on λMP in managed forest (Fig. 3c, d). There was no seasonal difference in the sensitivity of λMP to dispersal in natural forest. However, differing rates of dispersal between winter and summer in managed forest (i.e., early dispersing juveniles in winter but fewer non-breeders and breeders dispersing in winter) led to a seasonal change in the distribution of patches with high elasticities. Dispersal had a large influence on λMP in several managed forest territories, which was not explained by patch connectivity. Further, the identity of these influential territories (i.e., with high elasticities to dispersal rates) differed between seasons (Fig. 3c, d).
Metapopulation resilience
The damping ratio (ρ) for the metapopulation projection matrix (AMP) was 1.044 \(\left( {\frac{{\lambda_{1} }}{{\lambda_{2} }} = \frac{0.984}{{0.943}}} \right)\). ρ was higher (1.095) for the folded metapopulation matrix (\({\bf {A}}_{\mathrm{MP}}^{\mathrm{fold}}\)) because of a lower sub-dominant eigenvalue (λ2 = 0.899). Consequently, spatial structure reduced the damping ratio by 0.05, indicating that dispersal processes stabilised metapopulation dynamics. The damping ratio for the matrix including only natural forest territories (\(\rho ({\bf {A}}_{\mathrm{N}}^{\mathrm{fold}})\) = 1.118) was substantially lower than for the managed forest (\(\rho ({\bf {A}}_{\mathrm{M}}^{\mathrm{fold}})\) = 1.141). Consequently, population dynamics in natural forest are more resilient to a disturbance than in managed forest (see Appendix S5). However, the increase in ρ between the folded and standard matrices was similar for natural (\(\frac{\rho ({\bf {A}}_{\mathrm{N}}^{\mathrm{fold}})}{\rho ({\bf {A}}_{\mathrm{N}})}\) = 1.072) and managed forest (\(\frac{\rho ({\bf {A}}_{\mathrm{M}}^{\mathrm{fold}})}{\rho ({\bf {A}}_{\mathrm{M}})}\) = 1.075). Therefore, the stabilising effect of dispersal was similar for both forest types. Thus, the lower damping ratio in natural forest, compared to managed forest, was a result of higher survival and recruitment rates in natural forests.
Discussion
Successful conservation of species threatened by human activities requires modelling approaches accounting for local dynamics, spatial processes, and temporal variation. Here, we highlight the importance of these processes using a spatial population viability analysis. Forestry management reduced metapopulation persistence and stability in this forest-dwelling bird species. Furthermore, source-sink dynamics varied between forest types, and seasonally, resulting in spatiotemporal variation in the distribution of critical sites for metapopulation dynamics.
Metapopulation dynamics
Determining extinction risk and metapopulation persistence, and the role of local population dynamics and spatial processes in driving these, is key to the conservation of spatially structured populations (Hanski and Thomas 1994; Keymer et al. 2000; Kahilainen et al. 2018). For this boreal forest-dwelling species, metapopulation growth was stable i.e., overall abundances were neither substantially increasing nor declining over time. Thus, under current levels of forest management, this suggests that the metapopulation should not go extinct, while acknowledging the uncertainties in model parameters. However, when population dynamics were analysed separately for each forest, local population growth rates were stable in natural forest, but declining (λ < 1) in managed forest (Table 3). Negative population growth in commercially managed forests was a consequence of lower survival and recruitment. Forests under management have a less heterogeneous structure, leading to increased predation through hawks and owls, which detect prey more easily in more open forests (Griesser et al. 2017).
Under current forest management scenarios, the amount of high quality, pristine habitat will likely decrease further for Siberian jays (Bradter et al. unpublished). Here, we simulated a 25% reduction in natural forest territories, which indicated negative metapopulation growth. This emphasises the importance of retaining remaining pristine forest (e.g., by following more sustainable forestry practices, Spence 2001), to prevent population extinctions in the future.
As is common in longer-lived species, survival of breeding individuals was most influential on population growth and, consequently, was the main contributor to lower population growth rates in managed forest. Breeding individuals are also the dominant members in Siberian jay social groups, with higher survival than non-breeders on average (Griesser et al. 2017). Identifying life-history stages with a large influence on population growth can focus conservation measures. These results indicate that increasing forestry heterogeneity should have a substantial positive impact on population growth, largely via reduced breeder mortality. (Meta)population growth was less sensitive to changes in dispersal rates, compared to survival, transition and recruitment rates (Fig. 2).
Metapopulation stability
The damping ratio (ρ, i.e., ratio of the two largest eigenvalues), and similar concepts, (e.g., Day and Possingham 1995; Bode et al. 2008), has previously been used in a metapopulation context, e.g., as a measure of population-level connectivity (Shima et al. 2010). When applied to metapopulation projection matrices, as here, ρ provides a quantitative measure of population resilience, where a smaller damping ratio indicates that metapopulation growth is less affected by a disturbance in demographic and/or dispersal rates (see Appendix S5). Using this approach, we measured the role of dispersal in regulating metapopulation dynamics. Despite the comparatively low sensitivity of metapopulation growth to dispersal rates, our analysis of the damping ratio showed that this metapopulation was more resilient to a disturbance, i.e., changes in patch demography or dispersal, because of dispersal processes. Dispersal facilitates the re-allocation of individuals among social groups and reinforces a fixed social hierarchy including early and delayed dispersing juveniles, which consequently exhibit strongly contrasting individual fitness (Ekman et al. 2002; Griesser et al. 2014). In a metapopulation context, although reduced survival or reproduction in high-quality territories reduces metapopulation growth, this is, in part, compensated by poorer-quality territories taking over as ‘engines’ of metapopulation growth, through dispersal. Consequently, dispersal plays a stabilising role by dampening effects of a disturbance in critical, high-quality patches (Strasser et al. 2012). Since territories in natural forest had higher survival and recruitment rates, the resilience of population dynamics in natural forest to a disturbance was greater overall (i.e., a lower damping ratio). This indicates that population dynamics in natural forests are more resilient to a disturbance than in managed forests.
This novel use of damping ratios provides a measure of metapopulation resilience to e.g., local population extinctions, incorporating local dynamics and connectivity using a matrix population framework. This contributes to a better understanding of network properties and their role in mediating effects of (e.g., environmental) changes on metapopulation viability (Gaston et al. 2008). Quantifying metapopulation resilience (Hodgson et al. 2015) has been previously proposed as simple metric for conservation planning, to shift conservation focus from an individual- to multi-site perspective (Donaldson et al. 2019).
Source-sink dynamics
Source-sink dynamics reflect how differences in habitat quality affect vital rates and, ultimately, local population growth rates (Pulliam 1988). Source-sink dynamics are relatively common in spatially structured populations because of heterogeneity in habitat quality (Dias 1996). Forest-dwelling bird species often live in heterogeneous habitat and evidence of source-sink dynamics exists for such species (Pakkala et al. 2002; Nystrand et al. 2010; Vögeli et al. 2010). Forestry management reduces habitat quality, causing territories in managed forests to more often represent ‘sinks’ (Nystrand et al. 2010).
‘Patch value’ describes the contribution of local populations to metapopulation growth (Ovaskainen and Hanski 2003), which we measured here using territory-level elasticities (Ovaskainen and Hanski 2001, 2003). Overall, patch values were substantially higher in natural forest, compared to territories under forest management, because of the sensitivity of metapopulation growth to changes in demography in natural forests (Fig. 3). This emphasises the preservation of remaining pristine natural forest as a fundamental conservation goal to maintain metapopulation persistence. Furthermore, elasticity analysis showed that the influence of natural forest territories increased with territory connectivity, because they are more able to contribute individuals to surrounding patches (i.e., to act as source). Thus, in natural forest, isolated patches at the edges of the metapopulation contributed least to metapopulation persistence since dispersal is more limited, potentially increasing their extinction risk (Hanski and Ovaskainen 2000; Brito and Fernandez 2002). In managed forest, we identified few critical sites, where metapopulation growth was substantially (up to three times) more sensitive to changes in dispersal, than other managed forest territories. Since these critical sites contribute disproportionately to metapopulation growth, they could represent specific targets for conservation measures (Brito and Fernandez 2002). Preserving connectivity among patches (particularly allowing for dispersal from higher quality natural forest territories), would therefore also present a valuable conservation goal. However, as shown by the damping ratios, lower quality patches (i.e. sinks) can also act to buffer disturbance effects in more productive sites (Howe et al. 1991; Gill et al. 2001; Strasser et al. 2012). Asynchrony in local dynamics, ultimately limits variation in metapopulation growth, which increases metapopulation stability (Hanski 1998). Dispersal is therefore inherently important to facilitate buffering of disturbance effects, including further habitat degradation caused by commercial forestry. Nevertheless, demographic processes in natural forest territories were the main driver of metapopulation growth, while demography in managed forest had a far more minor impact on the growth rate. Conversely, dispersal in several managed forest territories made a large contribution to metapopulation growth, while dispersal in natural forest had a far lesser impact on the growth rate. Furthermore, the influence of dispersal on metapopulation growth in managed forest territories did not correlate with connectivity and there was greater variation among territories in their contributions than in natural forests.
Density dependence (density-dependent demography and dispersal) can be important in driving source-sink dynamics, e.g., where suppressed local recruitment causes a viable local population to be characterise as a sink (Watkinson and Sutherland 1995). The mechanisms by which density influences Siberian jay population dynamics are complex: higher densities positively effect survival and transition rates because of group vigilance. Density also positively effects recruitment rates but as an interactive effect with temperature, reflecting reduced resource competition in warming springs (Layton-Matthews et al. 2018). Due to the complex role of density-dependence, the metapopulation model formulated here was density independent.
Vital rates often vary seasonally, potentially affecting source-sink dynamics and thus metapopulation stability (Boughton 1999; Gill et al. 2001). In our study, seasonal variation in the contribution of demography on metapopulation growth rates was negligible. The sensitivity of λMP to dispersal in managed forests differed between seasons, likely due to higher dispersal rates in summer, although the contribution of this to metapopulation dynamics was small relative to demographic contributions. Patch values of territories in natural forest differed little between winter and summer, for both demography and dispersal. The minor role of seasonality (i.e., differences in vital rates between seasons) in driving metapopulation dynamics is likely explained by the dynamics of natural forest populations, which are largely driven by breeder survival that exhibited little variation across seasons. Ultimately, seasonality in demography and dispersal rates appear to play a somewhat minor role in the metapopulation dynamics of this particular species.
Conclusions
Our results show how forest management has reduced metapopulation growth and stability in a boreal forest species. Populations may therefore be increasingly vulnerable to further forestry effects and increasing temporal variability (e.g., due to climate change, Kahilainen et al. 2018). Overall, populations occupying natural forests have higher growth rates and are more inherently resilient to disturbance. Consequently, further loss of territories in natural forest could push such Siberian jay metapopulations towards extinction. Climate change has been shown to increase the difference in population trajectories in natural versus managed forests, thereby exacerbating the risk of metapopulation extinction (Layton-Matthews et al. 2018). These results therefore emphasise the necessity to protect remaining natural, old-growth, forests to conserve this species. Moreover, increasing structural heterogeneity in commercially managed forests should improve survival rates and thereby local population growth rates. Despite the apparent insensitivity of metapopulation growth to dispersal rates (based on perturbation analysis), dispersal plays a key role in metapopulation stability. It is therefore imperative to conserve both high-quality sites and maintain dispersal networks in both natural and managed forests. Our study also emphasises the value of studying eigenvalue distributions, particularly in the context of spatially structured populations.
The specificities of Siberian jay life history (e.g., stable territories, a rigid social hierarchy), have likely facilitated the development of such a detailed, spatial PVA. This may limit the generality of our findings to other species, even in boreal forests, as social structure and dispersal behaviour are rather unique to this species. Nevertheless, Siberian jays can, to some extent, be considered as an umbrella species, where the conservation-related recommendations here would likely benefit many other boreal species. Despite, the high-quality data requirements such PVAs have also been performed for other species (e.g., Ozgul et al. 2009). We emphasise the value in utilising increasingly advanced statistical tools to improve population viability assessments and ultimately make better predictions of wildlife population persistence in the face of ongoing human activities.
Data availability
Data used in this study will be submitted to Dryad.
References
Akçakaya HR (2000) Population viability analyses with demographically and spatially structured models. Ecol Bull 48:23–38
Akçakaya HR, McCarthy MA, Pearce JL (1995) Linking landscape data with population viability analysis: management options for the helmeted honeyeater Lichenostomus melanops cassidix. Biol Conserv 73:169–176. https://doi.org/10.1016/0006-3207(96)90068-3
Akçakaya HR, Atwood JL (1997) A habitat-based metapopulation model of the California Gnatcatcher. Conserv Biol 11:422–434. https://doi.org/10.1046/j.1523-1739.1997.96164.x
Behr DM, McNutt JW, Ozgul A, Cozzi G (2020) When to stay and when to leave? Proximate causes of dispersal in an endangered social carnivore. J Anim Ecol 89:2356–2366. https://doi.org/10.1111/1365-2656.13300
Bode M, Burrage K, Possingham HP (2008) Using complex network metrics to predict the persistence of metapopulations with asymmetric connectivity patterns. Ecol Model 214:201–209. https://doi.org/10.1016/j.ecolmodel.2008.02.040
Boughton DA (1999) Empirical evidence for complex source–sink dynamics with alternative states in a butterfly metapopulation. Ecology 80:2727–2739. https://doi.org/10.2307/177253
Boyce MS (1992) Population viability analysis. Annu Rev Ecol Syst 23:481–497. https://doi.org/10.1146/annurev.es.23.110192.002405
Brito D, Fernandez FA (2002) Patch relative importance to metapopulation viability: the neotropical marsupial Micoureus demerarae as a case study. Anim Conserv 5:45–51. https://doi.org/10.1017/s1367943002001063
Caswell H (1989) Analysis of life table response experiments I. Decomposition of effects on population growth rate. Ecol Model 46:221–237. https://doi.org/10.1016/0304-3800(89)90019-7
Caswell H (2001) Matrix population models. Sinauer Associates Inc., Sunderland, USA
Caswell H, Trevisan MC (1994) Sensitivity analysis of periodic matrix models. Ecology 75:1299–1303. https://doi.org/10.2307/1937455
Clark JS, Silman M, Kern R, Macklin E, HilleRisLambers J (1999) Seed dispersal near and far: patterns across temperate and tropical forests. Ecology 80:1475–1494. https://doi.org/10.1890/0012-9658(1999)080[1475:SDNAFP]2.0.CO;2
Coste CF, Austerlitz F, Pavard S (2017) Trait level analysis of multitrait population projection matrices. Theor Popul Biol 116:47–58. https://doi.org/10.1016/j.tpb.2017.07.002
Day JR, Possingham HP (1995) A stochastic metapopulation model with variability in patch size and position. Theor Popul Biol 48:333–360. https://doi.org/10.1006/tpbi.1995.1034
Demetrius L (1969) The sensitivity of population growth rate to pertubations in the life cycle components. Math Biosci 4:129–136. https://doi.org/10.1016/0025-5564(69)90009-1
Dias PC (1996) Sources and sinks in population biology. Trends Ecol Evol 11:326–330. https://doi.org/10.1016/0169-5347(96)10037-9
Doak D (1989) Spotted owls and old growth logging in the Pacific Northwest. Conserv Biol 3:389–396. https://doi.org/10.1111/j.1523-1739.1989.tb00244.x
Donaldson L, Bennie JJ, Wilson RJ, Maclean IM (2019) Quantifying resistance and resilience to local extinction for conservation prioritization. Ecol Appl 29:e01989. https://doi.org/10.1002/eap.1989
Ekman J, Griesser M (2002) Why offspring delay dispersal: experimental evidence for a role of parental tolerance. Proc R Soc Lond 269:1709–1713. https://doi.org/10.1098/rspb.2002.2082
Ekman J, Griesser M (2016) Siberian jays: delayed dispersal in absence of cooperative breeding. In: Koenig W, Dickinson J (eds) Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior. Cambridge University Press, Cambridge, UK, pp 6–18. https://doi.org/10.1017/cbo9781107338357.002
Ekman J, Bylin A, Tegelström H (1999) Increased lifetime reproductive success for Siberian jay (Perisoreus infaustus) males with delayed dispersal. Proc R Soc Lond 266:911–915. https://doi.org/10.1098/rspb.1999.0723
Ekman J, Eggers S, Griesser M (2002) Fighting to stay: the role of sibling rivalry for delayed dispersal. Anim Behav 64:453–459. https://doi.org/10.1006/anbe.2002.3075
Fagan WF, Meir E, Prendergast J, Folarin A, Karieva P (2001) Characterizing population vulnerability for 758 species. Ecol Lett 4:132–138. https://doi.org/10.1046/j.1461-0248.2001.00206.x
Figueira WF, Crowder LB (2006) Defining patch contribution in source-sink metapopulations: the importance of including dispersal and its relevance to marine systems. Popul Ecol 48:215–224. https://doi.org/10.1007/s10144-009-0173-1
Fretwell SD (1972) Populations in a seasonal environment. Princeton University Press, Princeton, USA. https://doi.org/10.2307/1296377
Fryxell JM (2001) Habitat suitability and source–sink dynamics of beavers. J Anim Ecol 70:310–316. https://doi.org/10.1111/j.1365-2656.2001.00492.x
Gaston KJ, Jackson SF, Cantú-Salazar L, Cruz-Piñón G (2008) The ecological performance of protected areas. Annu Rev Ecol Evol Syst 39:93–113. https://doi.org/10.1146/annurev.ecolsys.39.110707.173529
Gill JA, Norris K, Potts PM, Gunnarsson TG, Atkinson PW, Sutherland WJ (2001) The buffer effect and large-scale population regulation in migratory birds. Nature 412:436. https://doi.org/10.1016/s0169-5347(01)02340-0
Goodman LA (1971) On the sensitivity of the intrinsic growth rate to changes in the age-specific birth and death rates. Theor Popul Biol 2:339–354. https://doi.org/10.1016/0040-5809(71)90025-6
Griesser M, Lagerberg S (2012) Long-term effects of forest management on territory occupancy and breeding success of an open-nesting boreal bird species, the Siberian jay. Forest Ecol Manag 271:58–64. https://doi.org/10.1016/j.foreco.2012.01.037
Griesser M, Nystrand M, Ekman J (2006) Reduced mortality selects for family cohesion in a social species. Proc R Soc Lond 273:1881–1886. https://doi.org/10.1098/rspb.2006.3527
Griesser M, Nystrand M, Eggers S, Ekman J (2007) Impact of forestry practices on fitness correlates and population productivity in an open-nesting bird species. Conserv Biol 21:767–774. https://doi.org/10.1111/j.1523-1739.2007.00675.x
Griesser M, Halvarsson P, Sahlman T, Ekman J (2014) What are the strengths and limitations of direct and indirect assessment of dispersal? Insights from a long-term field study in a group-living bird species. Behav Ecol Sociobiol 68:485–497. https://doi.org/10.1007/s00265-013-1663-x
Griesser M, Halvarsson P, Drobniak SM, Vilà C (2015) Fine-scale kin recognition in the absence of social familiarity in the Siberian jay, a monogamous bird species. Mol Ecol 24:5726–5738. https://doi.org/10.1111/mec.13420
Griesser M, Mourocq E, Barnaby J, Bowgen KM, Eggers S, Fletcher K, Kozma R, Kurz F, Laurila A, Nystrand M (2017) Experience buffers extrinsic mortality in a group-living bird species. Oikos. https://doi.org/10.1111/oik.04098
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49. https://doi.org/10.1038/23876
Hanski IA, Gaggiotti OE (2004) Ecology, genetics and evolution of metapopulations. Elselvier Academic Press, London, UK. https://doi.org/10.1016/b978-0-12-323448-3.x5000-4
Hanski I, Thomas CD (1994) Metapopulation dynamics and conservation: a spatially explicit model applied to butterflies. Biol Cons 68:167–180. https://doi.org/10.1016/0006-3207(94)90348-4
Hanski, and Ovaskainen O, (2000) The metapopulation capacity of a fragmented landscape. Nature 404:755. https://doi.org/10.1038/35008063
Hastings A, Cuddington K, Davies KF, Dugaw CJ, Elmendorf S, Freestone A, Harrison S, Holland M, Lambrinos J, Malvadkar U (2005) The spatial spread of invasions: new developments in theory and evidence. Ecol Lett 8:91–101. https://doi.org/10.1111/j.1461-0248.2004.00687.x
Hodgson D, McDonald JL, Hosken DJ (2015) What do you mean, ‘resilient’? Trends Ecol Evol 30:503–506. https://doi.org/10.1016/j.tree.2015.06.010
Howe RW, Davis GJ, Mosca V (1991) The demographic significance of ‘sink’ populations. Biol Cons 57:239–255. https://doi.org/10.1016/0006-3207(91)90071-g
Hunter CM, Caswell H (2005) The use of the vec-permutation matrix in spatial matrix population models. Ecol Model 188:15–21. https://doi.org/10.1016/j.ecolmodel.2005.05.002
Imbeau L, Mönkkönen M, Desrochers A (2001) Long-term effects of forestry on birds of the eastern Canadian boreal forests: a comparison with Fennoscandia. Conserv Biol 15:1151–1162. https://doi.org/10.1046/j.1523-1739.2001.0150041151.x
Kahilainen A, van Nouhuys S, Schulz T, Saastamoinen M (2018) Metapopulation dynamics in a changing climate: Increasing spatial synchrony in weather conditions drives metapopulation synchrony of a butterfly inhabiting a fragmented landscape. Glob Change Biol 24:4316–4329. https://doi.org/10.1111/gcb.14280
Keymer JE, Marquet PA, Velasco-Hernández JX, Levin SA (2000) Extinction thresholds and metapopulation persistence in dynamic landscapes. Am Nat 156:478–494. https://doi.org/10.2307/3079052
Koons DN, Grand JB, Zinner B, Rockwell RF (2005) Transient population dynamics: relations to life history and initial population state. Ecol Model 185:283–297. https://doi.org/10.1016/j.ecolmodel.2004.12.011
Kot M, Schaffer WM (1984) The effects of seasonality on discrete models of population growth. Theor Popul Biol 26:340–360. https://doi.org/10.1016/0040-5809(84)90038-8
Lamberson RH, McKelvey R, Noon BR, Voss C (1992) A dynamic analysis of northern spotted owl viability in a fragmented forest landscape. Conserv Biol 6:505–512. https://doi.org/10.1046/j.1523-1739.1992.06040505.x
Lande R (1993) Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am Nat 142:911–927. https://doi.org/10.1086/285580
Layton-Matthews K, Ozgul A, Griesser M (2018) The interacting effects of forestry and climate change on the demography of a group-living bird population. Oecologia. https://doi.org/10.1007/s00442-018-4100-z
Lesnoff M, Ezanno P, Caswell H (2003) Sensitivity analysis in periodic matrix models: a postscript to Caswell and Trevisan. Math Comput Model 37:945–948. https://doi.org/10.2307/1937455
Moilanen A (2004) SPOMSIM: software for stochastic patch occupancy models of metapopulation dynamics. Ecol Model 179:533–550. https://doi.org/10.1016/j.ecolmodel.2004.04.019
Niemi G, Hanowski J, Helle P, Howe R, Mönkkönen M, Venier L, Welsh D (1998) Ecological sustainability of birds in boreal forests. Conserv Ecol. https://doi.org/10.5751/es-00079-020217
Noble IR, Dirzo R (1997) Forests as human-dominated ecosystems. Science 277:522–525. https://doi.org/10.1126/science.277.5325.522
Norris RD, Marra PP (2007) Seasonal interactions, habitat quality, and population dynamics in migratory birds. Condor 109:535–547. https://doi.org/10.1093/condor/109.3.535
Nystrand M, Griesser M, Eggers S, Ekman J (2010) Habitat-specific demography and source–sink dynamics in a population of Siberian jays. J Anim Ecol 79:266–274. https://doi.org/10.1111/j.1365-2656.2009.01627.x
Ovaskainen O, Hanski I (2001) Spatially structured metapopulation models: global and local assessment of metapopulation capacity. Theor Popul Biol 60:281–302. https://doi.org/10.1006/tpbi.2001.1548
Ovaskainen O, Hanski I (2003) How much does an individual habitat fragment contribute to metapopulation dynamics and persistence? Theor Popul Biol 64:481–495. https://doi.org/10.1016/s0040-5809(03)00102-3
Ozgul A, Oli MK, Armitage KB, Blumstein DT, Van Vuren DH (2009) Influence of local demography on asymptotic and transient dynamics of a yellow-bellied marmot metapopulation. Am Nat 173:517–530. https://doi.org/10.1086/597225
Pakkala T, Hanski I, Tomppo E (2002) Spatial ecology of the three-toed woodpecker in managed forest landscapes. Silva Fenn. https://doi.org/10.14214/sf.563
Paniw M, Maag N, Cozzi G, Clutton-Brock T, Ozgul A (2019) Life history responses of meerkats to seasonal changes in extreme environments. Science 363:631–635. https://doi.org/10.1126/science.aau5905
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132:652–661. https://doi.org/10.1086/284880
Rahmstorf S, Coumou D (2011) Increase of extreme events in a warming world. Proc Natl Acad Sci USA 108:17905–17909. https://doi.org/10.1073/pnas.1201163109
Shima JS, Noonburg EG, Phillips NE (2010) Life history and matrix heterogeneity interact to shape metapopulation connectivity in spatially structured environments. Ecology 91:1215–1224. https://doi.org/10.26686/wgtn.13012976
Spence JR (2001) The new boreal forestry: adjusting timber management to accommodate biodiversity. Trends Ecol Evol 16:591–593. https://doi.org/10.1016/s0169-5347(01)02335-7
Stenseth NC, Viljugrein H, Saitoh T, Hansen TF, Kittilsen MO, Bølviken E, Glöckner F (2003) Seasonality, density dependence, and population cycles in Hokkaido voles. Proc Natl Acad Sci USA 100:11478–11483. https://doi.org/10.2307/3546809
Strasser CA, Neubert MG, Caswell H, Hunter CM (2012) Contributions of high-and low-quality patches to a metapopulation with stochastic disturbance. Theor Ecol 5:167–179. https://doi.org/10.1007/s12080-010-0106-9
Thomas CD, Kunin WE (1999) The spatial structure of populations. J Anim Ecol 68:647–657. https://doi.org/10.1046/j.1365-2656.1999.00330.x
Thompson RM, Beardall J, Beringer J, Grace M, Sardina P (2013) Means and extremes: building variability into community-level climate change experiments. Ecol Lett 16:799–806. https://doi.org/10.1111/ele.12095
Tilman D, Clark M, Williams DR, Kimmel K, Polasky S, Packer C (2017) Future threats to biodiversity and pathways to their prevention. Nature 546:73–81. https://doi.org/10.1038/nature22900
Tischendorf L, Fahrig L (2000) On the usage and measurement of landscape connectivity. Oikos 90:7–19. https://doi.org/10.1034/j.1600-0706.2000.900102.x
Van Houtan KS, Pimm SL, Halley JM, Bierregaard RO Jr, Lovejoy TE (2007) Dispersal of Amazonian birds in continuous and fragmented forest. Ecol Lett 10:219–229. https://doi.org/10.1111/j.1461-0248.2007.01004.x
Vögeli M, Serrano D, Pacios F, Tella JL (2010) The relative importance of patch habitat quality and landscape attributes on a declining steppe-bird metapopulation. Biol Cons 143:1057–1067. https://doi.org/10.1016/j.biocon.2009.12.040
Watkinson AR, Sutherland WJ (1995) Sources, sinks and pseudo-sinks. J Anim Ecol 64:126–130. https://doi.org/10.2307/5833
Wisdom MJ, Mills LS, Doak DF (2000) Life stage simulation analysis: estimating vital-rate effects on population growth for conservation. Ecology 81:628–641. https://doi.org/10.2307/177365
Acknowledgements
We thank Folke Lindgren, Jan Ekman, Bohdan Sklepkovych, Sönke Eggers, Magdalena Nystrand, Jonathan Barnaby, Xenia Schleuning, Julian Klein and all volunteers for data collection; and Vidar Grøtan and Emma-Liina Marjakangas for advice on the manuscript. This study was supported by grants from Swiss National Science Foundation (MG: PPOOP3_123520, PP00P3_150752), ERA-Net BiodivERsA (AO, MG: 31BD30_172465d), the Swedish Research Council (MG), the National Science Centre, Poland, through EU Horizon 2020 (Marie Sklodowska-Curie grant 665778, MG), University of Zurich (AO, MG) and Norwegian University for Science and Technology (KLM, CFDC). CFDC was funded by Centre of Excellence grant from the Research Council of Norway (SFF-III: 223257).
Funding
Open access funding provided by Norwegian institute for nature research.
Author information
Authors and Affiliations
Contributions
MG collected the data. KLM analysed the data with assistance from AO and CFDC. KLM wrote the manuscript; MG and AO provided extensive editorial advice and CFDC provided comments on all versions of the manuscript.
Corresponding author
Additional information
Communicated by Christopher Whelan.
This paper shows how forestry management increases extinction risk and reduces stability in a bird metapopulation, using state-of-the-art and novel approaches.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Layton-Matthews, K., Griesser, M., Coste, C.F.D. et al. Forest management affects seasonal source-sink dynamics in a territorial, group-living bird. Oecologia 196, 399–412 (2021). https://doi.org/10.1007/s00442-021-04935-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-04935-6