Abstract
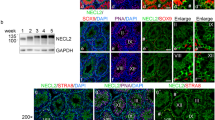
Communication between Sertoli cell is essential during spermatogenesis and testicular development in mice, and the dynamic balance of this communication is regulated by some adhesion proteins. In this study, we found that SPATA33 and CTNNA3 were involved in this process. Quantitative real-time PCR and western blotting showed similar trend of expression of two proteins in the testis of mice of different ages. Subsequently, CRISPR-Cas9 technique was used to prepare Spata33 knockout cell lines with TM4 cells, cell wound scratch assay showed that Spata33 gene knockout affected cell migration, and flow cytometry assay showed that Spata33 knockout resulted in a decreased percentage of G1 phase cells in TM4 cell line. In addition, phalloidin staining assay showed that Spata33 gene knockout disrupted the formation of F-actin. Moreover, the protein immunoprecipitation experiment showed the interaction between SPATA33 and CTNNA3, which affected the interaction between CTNNA3 and CTNNB1. SPATA33 inhibits the formation of CDH1-CTNNB1-CTNNA3 complex through its interaction with CTNNA3, thus weakening adhesion between Sertoli cell and promoting cell migration.








Similar content being viewed by others
Availability of data and material
All data and materials are transparent.
References
Andrews PW, Banting G, Damjanov I, Arnaud D, Avner P (1984) Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma 3(4):347-361
Bacchelli E et al (2014) A CTNNA3 compound heterozygous deletion implicates a role for αT-catenin in susceptibility to autism spectrum disorder. J Neurodev Disord 6:11
Barbara, Pascal de Santa et al (1998) Steroidogenic factor-1 regulates transcription of the human anti-mullerian hormone receptor. J Biolog Chem 273
Chang YF et al (2011) Role of beta-catenin in post-meiotic male germ cell differentiation. PLoS ONE 6(11):e28039
Chen H et al (2013) A novel testis-enriched gene Spata33 is expressed during spermatogenesis. PLoS ONE 8(7):e67882
Cheng CY, Mruk DD (2002) Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 82(4):825–874
Chojnacka K et al (2012) Expression of the androgen receptor in the testis of mice with a Sertoli cell specific knock-out of the connexin 43 gene (SCCx43KO(-/-)). Reprod Biol 12(4):341–346
Coates JC (2003) Armadillo repeat proteins: beyond the animal kingdom. Trends Cell Biol 13(9):463–471
Dijk M et al (2004) Differential downregulation of alphaT-catenin expression in placenta: trophoblast cell type-dependent imprinting of the CTNNA3 gene. Gene Expr Patterns 5(1):61–65
Du W et al (2016) Kinesin 1 drives autolysosome tubulation. Dev Cell 37(4):326–336
Dufour GK, Jannette M (2012) Cell lines: valuable tools or useless artifacts. Spermatogenesis 2(1):1–5
Fayomi AP, Orwig KE (2018) Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Research 29:207–214
Folmsbee SS et al (2016a) The cardiomyocyte protein alphaT-catenin contributes to asthma through regulating pulmonary vein inflammation. J Allergy Clin Immunol 138(1):123–129 e2
Folmsbee SS et al (2016b) alphaT-catenin in restricted brain cell types and its potential connection to autism. J Mol Psychiatry 4:2
Goossens S et al (2007) Truncated isoform of mouse alpha T-catenin is testis-restricted in expression and function. FASEB J 21(3):647–655
Han SP, Yap AS (2013) An alpha-catenin deja vu. Nat Cell Biol 15(3):238–239
Hermo L et al (2010a) Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 73(4):241–278
Hermo L et al (2010b) Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 3: developmental changes in spermatid flagellum and cytoplasmic droplet and interaction of sperm with the zona pellucida and egg plasma membrane. Microsc Res Tech 73(4):320–363
Janssens B et al (2001a) αT-Catenin: a novel tissue-specific β-catenin-binding protein mediating strong cell-cell adhesion. J Cell Sci 114
Janssens B et al (2001b) alpha T-Catenin: a novel tissue-specific beta-catenin-binding protein mediating strong cell-cell adhesion. J Cell Sci 114(17):3177–3188
Jiang G et al (2012) P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via beta-catenin and Kaiso respectively. PLoS ONE 7(1):e30303
Kast DJ, Dominguez R (2017) The cytoskeleton-autophagy connection. Curr Biol 27(8):R318–R326
Kim CH et al (2013) Overexpression of a novel regulator of p120 catenin, NLBP, promotes lung adenocarcinoma proliferation. Cell Cycle 12(15):2443–2453
Kuhn K, Romer W (2015) Considering autophagy, beta-Catenin and E-Cadherin as innovative therapy aspects in AML. Cell Death Dis 6:e1950
Li J et al (2015) Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ Res 116(1):70–79
Li J et al (2012) Loss of alphaT-catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia. J Cell Sci 125(Pt 4):1058–1067
Li Linxi et al (2017) Cell polarity, cell adhesion, and spermatogenesis: role of cytoskeletons. F1000Res 6:1565
Lie PP et al (2010) Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365(1546):1581–1592
Lorenzon A et al (2015) Homozygous desmocollin-2 mutations and arrhythmogenic cardiomyopathy. Am J Cardiol 116(8):1245–1251
Maiden SL, Hardin J (2011) The secret life of alpha-catenin: moonlighting in morphogenesis. J Cell Biol 195(4):543–552
McCabe MJ et al (2012) Androgen initiates Sertoli cell tight junction formation in the hypogonadal (hpg) mouse. Biol Reprod 87(2):38
Mi N et al (2015) CapZ regulates autophagosomal membrane shaping by promoting actin assembly inside the isolation membrane. Nat Cell Biol 17(9):1112–1123
Miyata H et al (2021) SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice. Proc Natl Acad Sci U S A 118(35):e2106673118
Monsef L et al (2018) Gene alterations and expression spectrum of SPATA33 in nonobstructive azoospermic Iranian men. Mol Reprod Dev 85(10):760–767
Moreau K et al (2015) Transcriptional regulation of annexin A2 promotes starvation-induced autophagy. Nat Commun 6:8045
Nong CZ et al (2006) P120ctn overexpression enhances beta-catenin-E-cadherin binding and down regulates expression of survivin and cyclin D1 in BEL-7404 hepatoma cells. World J Gastroenterol 12(8):1187–1191
Paul C, Robaire B (2013) Impaired function of the blood-testis barrier during aging is preceded by a decline in cell adhesion proteins and GTPases. PLoS ONE 8(12)
Sanjana NE, Shalem O, Zhang F (2014) Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11(8):783–784
Shahbazi MN, Perez-Moreno M (2015) Connections between cadherin-catenin proteins, spindle misorientation, and cancer. Tissue Barriers 3(3):e1045684
Shalem O et al (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343(6166):84–87
Shenying Fang et al (2020) Functional annotation of melanoma risk loci identifies novel susceptibility genes. Carcinogenesis 41(4)
Takeichi M (2014) Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 15(6):397–410
Tramoni M et al (2009) Contraceptive steroids from pharmaceutical waste perturbate junctional communication in Sertoli cells. Biochimie 91(11–12):1366–1375
Tyberghein K et al (2012) Tissue-wide overexpression of alpha-T-catenin results in aberrant trophoblast invasion but does not cause embryonic mortality in mice. Placenta 33(7):554–560
Vanpoucke G et al (2004) GATA-4 and MEF2C transcription factors control the tissue-specific expression of the alphaT-catenin gene CTNNA3. Nucleic Acids Res 32(14):4155–4165
Vite A, Li J, Radice GL (2015) New functions for alpha-catenins in health and disease: from cancer to heart regeneration. Cell Tissue Res 360(3):773–783
Wickline ED et al (2016) alphaT-Catenin is a constitutive actin-binding alpha-catenin that directly couples the cadherin.catenin complex to actin filaments. J Biol Chem 291(30):15687–15699
Wu X et al (2012) Transgene-mediated rescue of spermatogenesis in Cldn11-null mice. Biol Reprod 86(5):139, 1–11
Xu Xu et al (2021) SPATA33 functions as a mitophagy receptor in mammalian germline. Autophagy 17(5):1284–1286
Young JS et al (2009) Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod 80(1):162–174
Zhang X, Lui WY (2014) Dysregulation of nectin-2 in the testicular cells: an explanation of cadmium-induced male infertility. Biochim Biophys Acta 1839(9):873–884
Zhang Y et al (2021) SPATA33 is an autophagy mediator for cargo selectivity in germline mitophagy. Cell Death Differ 28:1076–1090
Funding
This work was supported by the National Key R&D Program of China (2019YFA0802500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All animal experiments and methods were performed in accordance with the relevant approved guidelines and regulations, as well as under the approval of the Ethics Committee of Wuhan University. This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
All data are permitted for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y. SPATA33 affects the formation of cell adhesion complex by interacting with CTNNA3 in TM4 cells. Cell Tissue Res 389, 145–157 (2022). https://doi.org/10.1007/s00441-022-03631-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03631-y