Abstract
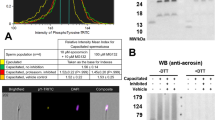
Defective mammalian spermatozoa are marked on their surface by proteolytic chaperone ubiquitin. To identify potential ubiquitinated substrates in the defective spermatozoa, we resolved bull sperm protein extracts on a two-dimensional gel and isolated a 64–65-kDa spot (p64) corresponding to one of the major ubiquitin-immunoreactive bands observed in the one-dimensional Western blots. Immune serum raised against this protein recognized a prominent, possibly glycosylated band/spot in the range of 55–68 kDa, consistent with the original spot used for immunization. Internal sequences obtained by Edman degradation of this spot matched the sequence of arylsulfatase A (ARSA), the sperm acrosomal enzyme thought to be important for fertility. By immunofluorescence, a prominent signal was detected on the acrosomal surface (boar and bull) and on the sperm tail principal piece (bull). A second immune serum raised against a synthetic peptide corresponding to an immunogenic internal sequence (GTGKSPRRTL) of the porcine ARSA also labeled sperm acrosome and principal piece. Both sera showed diminished immunoreactivity in the defective bull spermatozoa co-labeled with an anti-ubiquitin antibody. Western blotting and image-based flow cytometry (IBFC) confirmed a reduced ARSA immunoreactivity in the immotile sperm fraction rich in ubiquitinated spermatozoa. Larger than expected ARSA-immunoreactive bands were found in sperm protein extracts immunoprecipitated with anti-ubiquitin antibodies and affinity purified with matrix-bound, recombinant ubiquitin-binding UBA domain. These bands did not show the typical pattern of ARSA glycosylation but overlapped with bands preferentially binding the Lens culinaris agglutinin (LCA) lectin. By both epifluorescence microscopy and IBFC, the LCA binding was increased in the ubiquitinated spermatozoa with diminished ARSA immunoreactivity. ARSA was also found in the epididymal fluid suggesting that in addition to intrinsic ARSA expression in the testis, epididymal spermatozoa take up ARSA on their surface during the epididymal passage. We conclude that sperm surface ARSA is one of the ubiquitinated sperm surface glycoproteins in defective bull spermatozoa. Defective sperm surface thus differs from normal sperm surface by increased ubiquitination, reduced ARSA binding, and altered glycosylation.







Similar content being viewed by others
References
Baska KM, Manandhar G, Feng D, Agca Y, Tengowski MW, Sutovsky M, Yi YJ, Sutovsky P (2008) Mechanism of extracellular ubiquitination in the mammalian epididymis. J Cell Physiol 215(3):684–696
Belmonte SA, Ramano P, Sosa MA (2002) Compartmentalization of lysosomal enzymes in cauda epididymis of normal and castrated rats. Arch Androl 48(3):193–201
Brandon CIJ, Srivastava PN, Heusner GL, Fayrer-Hosken RA (1997) Extraction and quantification of acrosin, beta-N-acetylglucosaminidase, and arylsulfatase-A from equine ejaculated spermatozoa. J Exp Zool 279:301–308
Carmona E, Weerachatyanukul W, Soboloff T, Fluharty AL, White D, Promdee L, Ekker M, Berger T, Buhr M, Tanphaichitr N (2002a) Arylsulfatase A is present on the pig sperm surface and is involved in sperm-zona pellucida binding. Dev Biol 247(1):182–196
Carmona E, Weerachatyanukul W, Xu H, Fluharty A, Anupriwan A, Shoushtarian A, Chakrabandhu K, Tanphaichitr N (2002b) Binding of arylsulfatase A to mouse sperm inhibits gamete interaction and induces the acrosome reaction. Biol Reprod 66:1820–1827
Castellon EA, Balbontin JB (2000) Secretion of glycosidases in human epididymal cell cultures. Arch Androl 45(1):35–42
Cornwall GA (2014) Role of posttranslational protein modifications in epididymal sperm maturation and extracellular quality control. Adv Exp Med Biol 759:159–180
da Silveira JC, de Avila A, Garrett HL, Bruemmer JE, Winger QA, Bouma GJ (2018) Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J Endocrinol 236(1):R15–R27
Dacheux JL, Belleannee C, Guyonnet B, Labas V, Teixeira-Gomes AP, Ecroyd H, Druart X, Gatti JL, Dacheux F (2012) The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst Biol Reprod Med 58(4):197–210
Dudkiewicz A (1984) Purification of boar acrosomal arylsulfatase A and possible role in the penetration of cumulus cells. Biol Reprod 30(4):1005–1014
Edman P (1949) A method for the determination of amino acid sequence in peptides. Arch Biochem 22(3):475
Farooqui AA, Srivastava PN (1979) Isolation, characterization and the role of rabbit testicular arylsulphatase A in fertilization. Biochem J 181(2):331–337
Fraile B, Martin R, Miguel MPD, Arenas MI, Bethencourt FR, Peinado F, Paniagua R, Santamaria L (1996) Light and electron microscopic immunohistochemical localization of protein gene product 9.5 and ubiquitin immunoreactivities in the human epididymis and vas deferens. Biol. Reprod 55(2):291–297
Frenette G, Lessard C, Sullivan R (2002) Selected proteins of “prostasome-like particles” from epididymal cauda fluid are transferred to epididymal caput spermatozoa in bull. Biol Reprod 67(1):308–313
Gadella BM, Colenbrander B, LMv G, Lopes-Cardozo M (1993) Boar seminal vesicles secrete arylsulfatases into seminal plasma: evidence that desulfation of seminolipid occurs only after ejaculation. Biol Reprod 48(3):483–489
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82(2):373–428
Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Sprigs Harbor Laboratory, Cold Springs Harbor, New York
Hermo L (1995) Structural features and functions of principal cells of the intermediate zone of the epididymis of adult rats. Anat Rec 242(4):515–530
Hermo L, Jacks D (2002) Nature’s ingenuity: bypassing the classical secretory route via apocrine secretion. Mol Reprod Dev 63(3):394–410
Jones R (2004) Sperm survivial versus degradation in the mammalian epididymis: a hypothesis. Biol Reprod 71(5):1405–1411
Kaur SP, Chaudhry PS, Anand SR (1976) Acrosomal hydrolases in buffalo spermatozoa. Experientia 32(4):436–438
Kerns K, Zigo M, Drobnis EZ, Sutovsky M, Sutovsky P (2018) Zinc ion flux during mammalian sperm capacitation. Nat Commun 9(1):2061
Marini PE, Cabada MO (2003) One step purification and biochemical characterization of a spermatozoa-binding protein from porcine oviductal epithelial cells. Mol Reprod Dev 66(4):383–390
Molinari M, Calanca V, Galli C, Lucca P, Paganetti P (2003) Role of EDEM in the release of misfolded glycoproteins form the calnexin cycle. Science 299(5611):1397–1400
Ngernsoungnern A, Weerachatyanukul W, Saewu A, Thitilertdecha S, Sobhon P, Sretarugsa P (2004) Rat sperm AS-A: subcellular localization in testis and epididymis and surface distribution in epididymal sperm. Cell Tissue Res 318(2):353–363
Nikolajczyk BS, O'Rand MG (1992) Characterization of rabbit testis beta-galactosidase and arylsulfatase A: purification adn localization in spermatozoa during the acrosome reaction. Biol Reprod 46(3):366–378
Oda Y, Hosokawa N, Wada I, Nagata K (2003) EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science 299(5611):1394–1397
Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP (2003) A proteomics approach to understanding protein ubiquitination. Nat Biotechnol 21(8):921–926
Pickart CM (1998) Polyubiquitin chains. In: Peters J-M, Harris JR, Finley D (eds) Ubiquitin and the biology of the cell. Plenum Press, New York, pp 19–63
Rattanachaiyanont M, Weerachatyanukul W, Leveille M-C, Taylor T, D'Amours D, Rivers D, Leader A, Tanphaichitr N (2001) Anti-SLIP1-reactive proteins exist on human sperm and are involved in zona-pellucida binding. Mol Hum Reprod 7:633–640
Rosenkrans CF Jr, Zeng GQ, MC GT, Schoff PK, First NL (1993) Development of bovine embryos in vitro as affected by energy substrates. Biol Reprod 49(3):459–462
Santamaria L, Martin R, Paniagua R, Fraile B, Nistal M, Terenghi G, Polak JM (1993) Protein gene product 9.5 and ubiquitin immunoreactivities in rat epididymis epithelium. Histochemistry 100(2):131–138
Schenk M, Koppisetty CA, Santos DC, Carmona E, Bhatia S, Nyholm PG, Tanphaichitr N (2009) Interaction of arylsulfatase-A (ASA) with its natural sulfoglycolipid substrates: a computational and site-directed mutagenesis study. Glycoconj J 26(8):1029–1045
Song WH, Yi YJ, Sutovsky M, Meyers S, Sutovsky P (2016) Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc Natl Acad Sci U S A 113(36):E5261–E5270
Spiro RG (2004) Role of N-linked polymannose oligosaccharides in targeting glycoproteins for endoplasmic reticulum-associated degradation. Cell Mol Life Sci 61(9):1025–1041
Sullivan R (2016) Epididymosomes: role of extracellular microvesicles in sperm maturation. Front Biosci (Schol Ed) 8:106–114
Sutovsky P (2004) Visualization of sperm accessory structures in the mammalian spermatids, spermatozoa, and zygotes by immunofluorescence, confocal, and immunoelectron microscopy. Methods Mol Biol 253:59–77
Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, Shatten G (2001) A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J Cell Sci 15(Pt 1):5–7
Sutovsky P, Turner RM, Hameed AS, Sutovsky M (2003) Differential ubiquitination of stallion sperm proteins: possible implications for fertility and reproductive seasonality. Biol Reprod 68:688–698
Tantibhedhyangkul J, Weerachatyanukul W, Carmona E, Xu H, Anupriwan A, Michaud D, Tanphaichitr N (2002) Role of sperm surface arylsulfatase A in mouse sperm-zona pellucida binding. Biol Reprod 67:212–219
Thompson WE, Ramalho-Santos J, Sutovsky P (2003) Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol Reprod 69:254–260
Tulsiani DR (2003) Glycan modifying enzymes in luminal fluid of rat epididymis: are they involved in altering sperm surface glycoproteins during maturation? Microsc Res Tech 61(1):18–27
Wang Y, Penfold S, Tang X, Hattori N, Riley P, Harper JW, Cross JC, Tyers M (1999) Deletion of the Cu11 in mice causes arrest in early embryogenesis and accumulation of cyclin E. Curr Biol 9(20):1191–1194
Weerachatyanukul W, Xu H, Anupriwan A, Carmona E, Wade M, Hermo L, SMd S, Rippstein P, Sobhon P, Sretarugsa P, Tanphaichitr N (2003) Acquisition of arylsulfatase A onto the mouse sperm surface during epididymal transit. Biol Reprod 69:1183–1192
White D, Weerchatyanukul W, Gadella B, Kamolvarin N, Attar M, Tanphaichitr N (2000) Role of sperm sulfogalactosylglycerolipid in mouse sperm-zona pellucida binding. Biol Reprod 63:147–155
Wu A, Anupriwan A, Iamsaard S, Chakrabandhu K, Santos DC, Rupar T, Tsang BK, Carmona E, Tanphaichitr N (2007) Sperm surface arylsulfatase A can disperse the cumulus matrix of cumulus oocyte complexes. J Cell Physiol 213(1):201–211
Xu H, Liu F, Srakaew N, Koppisetty C, Nyholm PG, Carmona E, Tanphaichitr N (2012) Sperm arylsulfatase A binds to mZP2 and mZP3 glycoproteins in a nonenzymatic manner. Reproduction 144(2):209–219
Yamato K, Handa S, Yamakawa T (1974) Purification of arylsulfatase A from boar testis and its activities toward semiolipid and sulfatide. J Biochem (Tokyo) 75(6):1241–1247
Yang CH, Srivastava PN (1976) Purification and properties of arylsulphatase A from rabbit testis. Biochem J 159(1):133–142
Yi YJ, Manandhar G, Sutovsky M, Li R, Jonakova V, Oko R, Park CS, Prather RS, Sutovsky P (2007) Ubiquitin C-terminal hydrolase-activity is involved in sperm acrosomal function and anti-polyspermy defense during porcine fertilization. Biol Reprod 77(5):780–793
Yoshida Y, Chiba T, Tokunaga F, Kawasaki H, Iwai K, Suzuki T, Ito Y, Mutsuoka K, Yoshida M, Tanaka K, Tai T (2002) E3 ubiquitin ligase that recognizes sugar chains. Nature 418(6896):438–442
Yoshida Y, Mizushima T, Tanaka K (2019) Sugar-recognizing ubiquitin ligases: action mechanisms and physiology. Front Physiol 10:104
Zigo M, Jonakova V, Manaskova-Postlerova P (2011) Electrophoretic and zymographic characterization of proteins isolated by various extraction methods from ejaculated and capacitated boar sperms. Electrophoresis 32(11):1309–1318
Zimmerman SW, Manandhar G, Yi YJ, Gupta SK, Sutovsky M, Odhiambo JF, Powell MD, Miller DJ, Sutovsky P (2011) Sperm proteasomes degrade sperm receptor on the egg zona pellucida during mammalian fertilization. PLoS One 6(2):e17256
Acknowledgments
We thank Dr. Francis Pau (Oregon National Primate Research Center, Beaverton, OR) for help with rabbit immunization, Dr. Jan Pohl (Microchemical Facility, Emory University School of Medicine, Atlanta, GA) for peptide sequencing, Ms. Kathryn Craighead for manuscript editing, and Mr. Heinz Leigh and Ms. Nicole Leitman for technical assistance.
Funding
This work was in part supported grant #13324-2007 from The Missouri Life Sciences Research Board, National Research Initiative Competitive Grants no. 2007-35203-18274 and no. 2011-67015-20025 from the USDA National Institute of Food and Agriculture, Agriculture and Food Research Initiative Competitive Grant no. 2015-67015-23231 from the USDA NIFA, and seed funding from the F21C program of the University of Missouri-Columbia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All studies involving vertebrate animals were completed under the strict guidance of an Animal Care and Use protocol, and Antibody Production Protocol (included in the Supplementary data file) approved by the Animal Care and Use Committee (ACUC) of the University of Missouri. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kelsey, K.M., Zigo, M., Thompson, W.E. et al. Reciprocal surface expression of arylsulfatase A and ubiquitin in normal and defective mammalian spermatozoa. Cell Tissue Res 379, 561–576 (2020). https://doi.org/10.1007/s00441-019-03144-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03144-1