Abstract
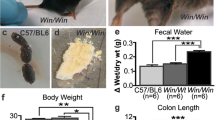
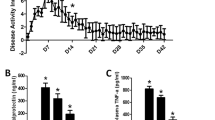
Rectal prolapse is associated with diminished anal sensitivity and rectal motor activity. Both sensory and motor functions are controlled by the extrinsic and intrinsic (enteric nervous system) innervation of the gastrointestinal tract. Studies of changes in intestinal innervation in humans and in animal models with rectal prolapse are extremely scarce. The Winnie mouse model of spontaneous chronic colitis closely represents human inflammatory bowel disease and is prone to develop rectal prolapse. We have investigated changes in the myenteric and inhibitory motor neurons and evaluated changes in the density of sensory afferent, sympathetic, and parasympathetic fibers in the rectal colon of Winnie mice with and without rectal prolapse. Our results demonstrate that rectal prolapse in Winnie mice with chronic colitis is correlated with enhanced levels of inflammation, gross morphological damage, and muscular hypertrophy of the rectum. Animals with prolapse have more severe damage to the rectal innervation compared with Winnie mice without prolapse. This includes more severe neuronal loss in the myenteric plexus, involving a decrease in nNOS-immunoreactive neurons (not observed in Winnie mice without prolapse) and a more pronounced loss of VAChT-immunoreactive fibers. Both Winnie mice with and without prolapse have comparable levels of noradrenergic and sensory fiber loss in the rectum. This is the first study providing evidence that the damage and death of enteric neurons, including nitrergic neurons in myenteric ganglia and the loss of cholinergic nerve fibers, are important factors in structural changes in the rectum of mice with rectal prolapse.







Similar content being viewed by others
References
Antao B, Bradley V, Roberts JP, Shawis R (2005) Management of rectal prolapse in children. Dis Colon Rectum 48:1620–1625
Assas BM, Pennock JI, Miyan JA (2014) Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci 8:23
Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G (2009) Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil 21:746–e46
Boyer L, Ghoreishi M, Templeman V, Vallance BA, Buchan AM, Jevon G, Jacobson K (2005) Myenteric plexus injury and apoptosis in experimental colitis. Auton Neurosci 117:41–53
Brookes SJ (2001) Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 262:58–70
Brumovsky PR, La JH, Gebhart GF (2014) Distribution across tissue layers of extrinsic nerves innervating the mouse colorectum—an in vitro anterograde tracing study. Neurogastroenterol Motil 26:1494–1507
Cervi AL, Lukewich MK, Lomax AE (2014) Neural regulation of gastrointestinal inflammation: role of the sympathetic nervous system. Auton Neurosci 182:83–88
Choi S, Parajuli SP, Yeum CH, Park CG, Kim MY, Kim YD, Cha KH, Park YB, Park JS, Jeong HS, Jun JY (2008) Calcitonin gene-related peptide suppresses pacemaker currents by nitric oxide/cGMP-dependent activation of ATP-sensitive K(+) channels in cultured interstitial cells of Cajal from the mouse small intestine. Mol Cell 26:181–185
de Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE (2005) Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut 54:1107–1113
de Souza RR, Moratelli HB, Borges N, Liberti EA (1993) Age-induced nerve cell loss in the myenteric plexus of the small intestine in man. Gerontology 39:183–188
de Tayrac R, Sentilhes L (2013) Complications of pelvic organ prolapse surgery and methods of prevention. Int Urogynecol J 24:1859–1872
Depoortere I, Thijs T, Peeters TL (2002) Generalized loss of inhibitory innervation reverses serotonergic inhibition into excitation in a rabbit model of TNBS-colitis. Br J Pharmacol 135:2011–2019
Domoto T, Yang H, Bishop AE, Polak JM, Oki M (1992) Distribution and origin of extrinsic nerve fibers containing calcitonin gene-related peptide, substance P and galanin in the rat upper rectum. Neurosci Res 15:64–73
Eri RD, Adams RJ, Tran TV, Tong H, Das I, Roche DK, Oancea I, Png CW, Jeffery PL, Radford-Smith GL, Cook MC, Florin TH, McGuckin MA (2011) An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol 4:354–364
Eysselein VE, Reinshagen M, Cominelli F, Sternini C, Davis W, Patel A, Nast CC, Bernstein D, Anderson K, Khan H, Snape WJ (1991) Calcitonin gene-related peptide and substance P decrease in the rabbit colon during colitis. A time study. Gastroenterology 101:1211–1219
Eysselein VE, Reinshagen M, Patel A, Davis W, Nast C, Sternini C (1992) Calcitonin gene-related peptide in inflammatory bowel disease and experimentally induced colitis. Ann N Y Acad Sci 657:319–327
Farouk R, Duthie GS (1998) Rectal prolapse and rectal invagination. Eur J Surg 164:323–332
Felt-Bersma RJ, Poen AC, Cuesta MA, Meuwissen SG (1997) Anal sensitivity test: what does it measure and do we need it? Cause or derivative of anorectal complaints. Dis Colon Rectum 40:811–816
Fox A, Tietze PH, Ramakrishnan K (2014) Anorectal conditions: rectal prolapse. FP Essent 419:28–34
Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9:286–294
Goyal RK (2013) Revised role of interstitial cells of Cajal in cholinergic neurotransmission in the gut. J Physiol (Lond) 591:5413–5414
Grider JR (2003) Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther 307:460–467
Hammond K, Beck DE, Margolin DA, Whitlow CB, Timmcke AE, Hicks TC (2007) Rectal prolapse: a 10-year experience. Ochsner J 7:24–32
Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Chin WP, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin THJ, Goodnow CC, McGuckin MA (2008) Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 5:e54
Jacobs LK, Lin YJ, Orkin BA (1997) The best operation for rectal prolapse. Surg Clin North Am 77:49–70
Jonsson M, Norrgard O, Forsgren S (2007) Presence of a marked nonneuronal cholinergic system in human colon: study of normal colon and colon in ulcerative colitis. Inflamm Bowel Dis 13:1347–1356
Kairaluoma MV, Kellokumpu IH (2005) Epidemiologic aspects of complete rectal prolapse. Scand J Surg 94:207–210
Kajimoto Y, Hashimoto T, Shirai Y, Nishino N, Kuno T, Tanaka C (1992) cDNA cloning and tissue distribution of a rat ubiquitin carboxyl-terminal hydrolase PGP9.5. J Biochem 112:28–32
Kim DS, Tsang CB, Wong WD, Lowry AC, Goldberg SM, Madoff RD (1999) Complete rectal prolapse: evolution of management and results. Dis Colon Rectum 42:460–469
Kono T, Chisato N, Ebisawa Y, Asama T, Sugawara M, Ayabe T, Kohgo Y, Kasai S, Yoneda M, Takahashi T (2004) Impaired nitric oxide production of the myenteric plexus in colitis detected by a new bioimaging system. J Surg Res 117:329–338
Kressel M, Berthoud HR, Neuhuber WL (1994) Vagal innervation of the rat pylorus: an anterograde tracing study using carbocyanine dyes and laser scanning confocal microscopy. Cell Tissue Res 275:109–123
Kumar MJM, Nagarajan P, Venkatesan R, Juyal RC (2004) Case report and short communication: rectal prolapse associated with an unusual combination of pinworms and citrobacter species infection in FVB mice colony. Scand J Lab Anim Sci 31:221–223
Lecci A, Santicioli P, Maggi CA (2002) Pharmacology of transmission to gastrointestinal muscle. Curr Opin Pharmacol 2:630–641
Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD (2004) Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci 24:1330–1339
Lies B, Beck K, Keppler J, Saur D, Groneberg D, Friebe A (2015) Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J Physiol (Lond) 593:4589–4601
Lin A, Lourenssen S, Stanzel RDP, Blennerhassett MG (2005) Selective loss of NGF-sensitive neurons following experimental colitis. Exp Neurol 191:337–343
Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM (2005) Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil 17:751–760
Lomax AE, Sharkey KA, Furness JB (2010) The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil 22:7–18
Lourenssen S, Wells RW, Blennerhassett MG (2005) Differential responses of intrinsic and extrinsic innervation of smooth muscle cells in rat colitis. Exp Neurol 195:497–507
Luckensmeyer GB, Keast JR (1998) Projections of pelvic autonomic neurons within the lower bowel of the male rat: an anterograde labelling study. Neuroscience 84:263–280
Madiba TE, Baig MK, Wexner SD (2005) Surgical management of rectal prolapse. Arch Surg 140:63–73
Marceau C, Parc Y, Debroux E, Tiret E, Parc R (2005) Complete rectal prolapse in young patients: psychiatric disease a risk factor of poor outcome. Colorectal Dis 7:360–365
Matsumoto K, Hosoya T, Tashima K, Namiki T, Murayama T, Horie S (2011) Distribution of transient receptor potential vanilloid 1 channel-expressing nerve fibers in mouse rectal and colonic enteric nervous system: relationship to peptidergic and nitrergic neurons. Neuroscience 172:518–534
Matteoli G, Boeckxstaens GE (2013) The vagal innervation of the gut and immune homeostasis. Gut 62:1214–1222
McGuckin MA, Eri RD, Das I, Lourie R, Florin TH (2011) Intestinal secretory cell ER stress and inflammation. Biochem Soc Trans 39:1081–1085
McIntyre AS, Thompson DG (1992) Review article: adrenergic control of motor and secretory function in the gastrointestinal tract. Aliment Pharmacol Ther 6:125–142
Miampamba M, Sharkey KA (1998) Distribution of calcitonin gene-related peptide, somatostatin, substance P and vasoactive intestinal polypeptide in experimental colitis in rats. Neurogastroenterol Motil 10:315–329
Miampamba M, Chery-Croze S, Chayvialle JA (1992) Spinal and intestinal levels of substance P, calcitonin gene-related peptide and vasoactive intestinal polypeptide following perendoscopic injection of formalin in rat colonic wall. Neuropeptides 22:73–80
Miller CL, Muthupalani S, Shen Z, Fox JG (2014) Isolation of Helicobacter spp. from mice with rectal prolapses. Comp Med 64:171–178
Moszkowicz D, Peschaud F, Bessede T, Benoit G, Alsaid B (2012) Internal anal sphincter parasympathetic-nitrergic and sympathetic-adrenergic innervation: a 3-dimensional morphological and functional analysis. Dis Colon Rectum 55:473–481
Nagatsu T (1989) The human tyrosine hydroxylase gene. Cell Mol Neurobiol 9:313–321
Nurgali K, Nguyen TV, Matsuyama H, Thacker M, Robbins HL, Furness JB (2007) Phenotypic changes of morphologically identified guinea-pig myenteric neurons following intestinal inflammation. J Physiol (Lond) 583:593–609
Nurgali K, Qu Z, Hunne B, Thacker M, Pontell L, Furness JB (2011) Morphological and functional changes in guinea-pig neurons projecting to the ileal mucosa at early stages after inflammatory damage. J Physiol (Lond) 589:325–339
O’Brien DP (2007) Rectal prolapse. Clin Colon Rectal Surg 20:125–132
Olsson C, Chen BN, Jones S, Chataway TK, Costa M, Brookes SJ (2006) Comparison of extrinsic efferent innervation of guinea pig distal colon and rectum. J Comp Neurol 496:787–801
Pelletier AM, Venkataramana S, Miller KG, Bennett BM, Nair DG, Lourenssen S, Blennerhassett MG (2010) Neuronal nitric oxide inhibits intestinal smooth muscle growth. Am J Physiol Gastrointest Liver Physiol 298:G896–G907
Qu Z, Apel ED, Doherty CA, Hoffman PW, Merlie JP, Huganir RL (1996) The synapse-associated protein rapsyn regulates tyrosine phosphorylation of proteins colocalized at nicotinic acetylcholine receptor clusters. Mol Cell Neurosci 8:171–184
Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB (2008) Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res 334:147–161
Rahman AA, Robinson AM, Jovanovska V, Eri R, Nurgali K (2015) Alterations in the distal colon innervation in Winnie mouse model of spontaneous chronic colitis. Cell Tissue Res 362:497–512
Rattan S, Regan RF, Patel CA, De Godoy MA (2005) Nitric oxide not carbon monoxide mediates nonadrenergic noncholinergic relaxation in the murine internal anal sphincter. Gastroenterology 129:1954–1966
Renzi D, Mantellini P, Calabro A, Panerai C, Amorosi A, Paladini I, Salvadori G, Garcea MR, Surrenti C (1998) Substance P and vasoactive intestinal polypeptide but not calcitonin gene-related peptide concentrations are reduced in patients with moderate and severe ulcerative colitis. Ital J Gastroenterol Hepatol 30:62–70
Ridolfi TJ, Tong WD, Takahashi T, Kosinski L, Ludwig KA (2009) Sympathetic and parasympathetic regulation of rectal motility in rats. J Gastrointest Surg 13:2027–2033
Rivera LR, Poole DP, Thacker M, Furness JB (2011) The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil 23:980–988
Rogala AR, Morgan AP, Christensen AM, Gooch TJ, Bell TA, Miller DR, Godfrey VL, de Villena FP (2014) The collaborative cross as a resource for modeling human disease: CC011/Unc, a new mouse model for spontaneous colitis. Mamm Genome 25:95–108
Sainio AP, Voutilainen PE, Husa AI (1991) Recovery of anal sphincter function following transabdominal repair of rectal prolapse: cause of improved continence? Dis Colon Rectum 34:816–821
Sanders KM, Hwang SJ, Ward SM (2010) Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol (Lond) 588:4621–4639
Sanovic S, Lamb DP, Blennerhassett MG (1999) Damage to the enteric nervous system in experimental colitis. Am J Pathol 155:1051–1057
Sharrad DF, Hibberd TJ, Kyloh MA, Brookes SJ, Spencer NJ (2015) Quantitative immunohistochemical co-localization of TRPV1 and CGRP in varicose axons of the murine oesophagus, stomach and colorectum. Neurosci Lett 599:164–171
Snooks SJ, Henry MM, Swash M (1985) Anorectal incontinence and rectal prolapse: differential assessment of the innervation to puborectalis and external anal sphincter muscles. Gut 26:470–476
Spencer NJ, Smith TK (2001) Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol (Lond) 533:787–799
Straub RH, Grum F, Strauch U, Capellino S, Bataille F, Bleich A, Falk W, Scholmerich J, Obermeier F (2008) Anti-inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut 57:911–921
Taffs LF (1976) Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13
Terauchi A, Kobayashi D, Mashimo H (2005) Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol 289:G291–G299
Wafai L, Taher M, Jovanovska V, Bornstein JC, Dass CR, Nurgali K (2013) Effects of oxaliplatin on mouse myenteric neurons and colonic motility. Front Neurosci 7:1–8
Wakabayashi K, Takahashi H, Ohama E, Ikuta F (1989) Tyrosine hydroxylase-immunoreactive intrinsic neurons in the Auerbach’s and Meissner’s plexuses of humans. Neurosci Lett 96:259–263
Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM (2000) Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 20:1393–1403
Weihe E, Tao-Cheng JH, Schäfer MKH, Erickson JD, Eiden LE (1996) Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci U S A 93:3547–3552
Zaitouna M, Alsaid B, Diallo D, Benoit G, Bessede T (2013) Identification of the origin of adrenergic and cholinergic nerve fibers within the superior hypogastric plexus of the human fetus. J Anat 223:14–21
Zhao A, Bossone C, Pineiro-Carrero V, Shea-Donohue T (2001) Colitis-induced alterations in adrenergic control of circular smooth muscle in vitro in rats. J Pharmacol Exp Ther 299:768–774
Zorenkov D, Otto S, Bottner M, Hedderich J, Vollrath O, Ritz JP, Buhr H, Wedel T (2011) Morphological alterations of the enteric nervous system in young male patients with rectal prolapse. Int J Colorectal Dis 26:1483–1491
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript do not have any potential conflicts to disclose.
Role of authors
AAR and AMR performed experiments, analyzed data, and wrote the manuscript. KN, RE, and SJHB developed the concept, obtained funding, and edited manuscript. KN supervised the study.
Additional information
Ahmed A. Rahman and Ainsley M. Robinson contributed equally to this work.
This study was supported by the Australian National Health & Medical Research Council project grant 1032414 and a Victoria University research support grant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
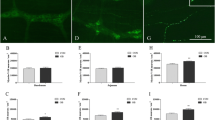
Changes in the innervation of the distal colon (non-prolapsed regions). Total number of myenteric neurons (a), nNOS-IR neurons (b), and VAChT-IR fibers (c) in cross sections, of VAChT-IR fibers in wholemount preparations (d), of TH-IR fibers in wholemount preparations (e), and of CGRP-IR fibers in cross sections (f) of the distal colon (non-prolapsed regions) of Winnie-prolapse mice compared with data from the same regions of the distal colon from Winnie and C57/BL6 mice (Rahman et al. 2015). (GIF 79 kb)
Rights and permissions
About this article
Cite this article
Rahman, A.A., Robinson, A.M., Brookes, S.J.H. et al. Rectal prolapse in Winnie mice with spontaneous chronic colitis: changes in intrinsic and extrinsic innervation of the rectum. Cell Tissue Res 366, 285–299 (2016). https://doi.org/10.1007/s00441-016-2465-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2465-z