Abstract
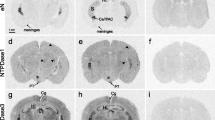
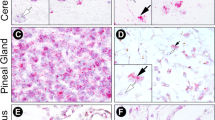
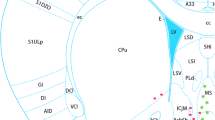
Ecto-5′-nucleotidase (eN) is the major extracellular adenosine-producing ecto-enzyme in mouse brain. Via the production of adenosine, eN participates in many physiological and pathological processes, such as wakefulness, inflammation, nociception and neuroprotection. The mechanisms regulating the expression of eN are therefore of considerable neurobiological and clinical interest. Having previously described a modulatory effect of melatonin in the regulation of eN mRNA levels, we decided to analyze the melatonin receptor subtype involved in the regulation of eN mRNA levels by comparing eN mRNA patterns in melatonin-proficient transgenic mice lacking either the melatonin receptor subtype 1 (MT1 KO) or both melatonin receptor subtypes (MT1 and MT2; MT1/2 KO) with the corresponding melatonin-proficient wild-type (WT) controls. By means of radioactive in situ hybridization, eN mRNA levels were found to be diminished in both MT1 and MT1/2 KO mice compared with WT controls suggesting stimulatory impacts of melatonin receptors on eN mRNA levels. Whereas eN mRNA levels increased during the day and peaked at night in WT and MT1 KO mice, eN mRNA levels at night were reduced and the peak was shifted toward day-time in double MT1/2 KO mice. These data suggest that the MT2 receptor subtype may play a role in the temporal regulation of eN mRNA availability. Notably, day-time locomotor activity was significantly higher in MT1/2 KO compared with WT mice. Our results suggest melatoninergic signaling as an interface between the purinergic system and the circadian system.




Similar content being viewed by others
References
Ackermann H (1991) BIAS. A program package for biometrical analysis of samples. Comput Stat Data Anal 11:223–224
Adamah-Biassi E, Zhang Y, Jung H, Vissapragada S, Miller R, Dubocovich M (2013) Distribution of MT1 melatonin receptor promoter-driven RFP expression in the brains of BAC C3H/HeN transgenic mice. J Histochem Cytochem 62:70–84
Alam M, Costales M, Cavanaugh C, Williams K (2015) Extracellular adenosine generation in the regulation of pro-inflammatory responses and pathogen colonization. Biomolecules 5:775–792
Allard B, Turcotte M, Stagg J (2012) CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol 15:1–8
Augusto E, Matos M, Sevigny J, El-Tayeb A, Bynoe MS, Muller CE, Cunha RA, Chen J (2013) Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci 33:11390–11399
Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD (1993) The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res 15:161–190
Bhattacharya A, Vavra V, Svobodova I, Bendova Z, Vereb G, Zemkova H (2013) Potentiation of inhibitory synaptic transmission by extracellular ATP in rat suprachiasmatic nuclei. J Neurosci 33:8035–44
Boison D (2008) Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol 8:2–7
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187
Burnstock G (2015) An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology. doi:10.1016/j.neuropharm.2015.05.031
Chen L, He X, Zhang Y, Chen X, Lai X, Shao J, Shi Y, Zhou N (2014) Melatonin receptor type 1 signals to extracellular signal-regulated kinase 1 and 2 via Gi and Gs dually coupled pathways in HEK-293 cells. Biochemistry 53:2827–2839
Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2:351–360
Comai S, Gobbi G (2014) Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci 39:6–21
Doolen S, Krause DN, Dubocovich ML, Duckles SP (1998) Melatonin mediates two distinct responses in vascular smooth muscle. Eur J Pharmacol 345:67–69
Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J (2010) International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 62:343–380
Elliott KJ, Todd Weber E, Rea MA (2001) Adenosine A1 receptors regulate the response of the hamster circadian clock to light. Eur J Pharmacol 414:45–53
Ena SL, De Backer J, Schiffmann SN, Kerchove d'Exaerde A de (2013) FACS array profiling identifies ecto-5′ nucleotidase as a striatopallidal neuron-specific gene involved in striatal-dependent learning. J Neurosci 33:8794–8809
Esposito E, Cuzzocrea S (2010) Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol 8:228–242
Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B, Ysrraelit MC, Zhu C, Kuchroo VK, Rabinovich GA, Quintana FJ, Correale J (2015) Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162:1338–1352
Fisher SP, Sugden D (2009) Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett 457:93–96
Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates. Academic Press, New York
Gall C von, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger P, Weaver DR, Korf HW, Hastings MH, Stehle JH (2002a) Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci 5:234–238
Gall C von, Stehle JH, Weaver DR (2002b) Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 309:151–162
Hallworth R, Cato M, Colbert C, Rea MA (2002) Presynaptic adenosine A1 receptors regulate retinohypothalamic neurotransmission in the hamster suprachiasmatic nucleus. J Neurobiol 52:230–240
Hansen KR, Resta R, Webb CF, Thompson LF (1995) Isolation and characterization of the promoter of the human 5′-nucleotidase (CD73)-encoding gene. Gene 167:307–312
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin-a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93:350–384
Haskó G, Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25:33–39
Homola M, Pfeffer M, Fischer C, Zimmermann H, Robson SC, Korf HW (2015) Expression of ectonucleotidases in the prosencephalon of melatonin-proficient C3H and melatonin-deficient C57Bl mice: spatial distribution and time-dependent changes. Cell Tissue Res 362:163–176
Huang Z, Zhang Z, Qu W (2014) Roles of adenosine and its receptors in sleep–wake regulation. In: Mori A (ed) Adenosine receptors in neurology and psychiatry, vol 119. Elsevier, Amsterdam, pp 349–371
Jacobson KA, Gao Z (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5:247–264
Jan JE, Reiter RJ, Wong PKH, Bax MCO, Ribary U, Wasdell MB (2011) Melatonin has membrane receptor-independent hypnotic action on neurons: an hypothesis. J Pineal Res 50:233–240
Korf HW, Gall C von (2013) Circadian physiology. In: Pfaff DW (ed) Neuroscience in the 21st century. From basic to clinical. Circadian physiology. Springer, Berlin Heidelberg New York, pp 1813–1845
Kulesskaya N, Võikar V, Peltola M, Yegutkin GG, Salmi M, Jalkanen S, Rauvala H (2013) CD73 is a major regulator of adenosinergic signalling in mouse brain. PLoS One 8:e66896
Lacoste B, Angeloni D, Dominguez-Lopez S, Calderoni S, Mauro A, Fraschini F, Descarries L, Gobbi G (2015) Anatomical and cellular localization of melatonin MT 1 and MT 2 receptors in the adult rat brain. J Pineal Res 58:397–417
Langer D, Hammer K, Koszalka P, Schrader J, Robson SC, Zimmermann H (2008) Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res 334:199–217
Legros C, Devavry S, Caignard S, Tessier C, Delagrange P, Ouvry C, Boutin JA, Nosjean O (2014) Melatonin MT 1 and MT 2 receptors display different molecular pharmacologies only in the G-protein coupled state. Br J Pharmacol 171:186–201
Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19:91–102
Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J (2012) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J Pineal Res 54:1–14
Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual R, Shiromani P (2004) The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience 123:361–370
Narravula S, Lennon PF, Mueller BU, Colgan SP (2000) Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol 165:5262–5268
Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, Rivara S, Bedini A, Angeloni D, Fraschini F, Mor M, Tarzia G, Descarries L, Gobbi G (2011) Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci 31:18439–18452
Perez-Aso M, Feig JL, Aránzazu M, Cronstein BN (2013) Adenosine A2A receptor and TNF-α regulate the circadian machinery of the human monocytic THP-1 cells. Inflammation 36:152–162
Pfeffer M, Rauch A, Korf HW, Gall C von (2012) The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol Int 29:415–429
Regateiro FS, Cobbold SP, Waldmann H (2013) CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol 171:1–7
Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology (Bethesda) 29:325–333
Shammah-Lagnado SJ, Alheid GF, Heimer L (2001) Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol 439:104–126
Shiu SYW, Pang B, Tam CW, Yao K (2010) Signal transduction of receptor-mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of Gαs and Gαq proteins. J Pineal Res 49:301–311
Sigworth L, Rea M (2003) Adenosine A1 receptors regulate the response of the mouse circadian clock to light. Brain Res 960:246–251
Spychala J, Kitajewski J (2004) Wnt and β-catenin signaling target the expression of ecto-5′-nucleotidase and increase extracellular adenosine generation. Exp Cell Res 296:99–108
Srinivasan V, Lauterbach EC, Ho KY, Acuña-Castroviejo D, Zakaria R, Brzezinski A (2012) Melatonin in antinociception: its therapeutic applications. Curr Neuropharmacol 10:167–178
Tosini G, Owino S, Guillaume J, Jockers R (2014) Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. Bioessays 36:778–787
Uz T, Akhisaroglu M, Ahmed R, Manev H (2003) The pineal gland is critical for circadian period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology 28:2117–2123
Vacas MI, Sarmiento MI, Pereyra EN, Cardinali DP (1989) Effect of adenosine on melatonin and norepinephrine release in rat pineal explants. Acta Physiol Pharmacol Latinoam 39:189–195
Wan Q, Man H, Liu F, Braunton J, Niznik HB, Pang SF, Brown GM, Wang YT (1999) Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci 2:401–403
Wang X (2009) The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther 15:345–357
Wang H, Lee S, Lo Nigro C, Lattanzio L, Merlano M, Monteverde M, Matin R, Purdie K, Mladkova N, Bergamaschi D, Harwood C, Syed N, Szlosarek P, Briasoulis E, McHugh A, Thompson A, Evans A, Leigh I, Fleming C, Inman GJ, Hatzimichael E, Proby C, Crook T (2012) NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. Br J Cancer 106:1446–1452
Zawilska JB, Skene DJ, Arendt J (2009) Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep 61:383–410
Zimmermann H (1992) 5′-Nucleotidase molecular structure and functional aspects. Biochem J 285:345–365
Zylka MJ (2011) Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med 17:188–196
Acknowledgments
The authors thank Elke Laedtke and Iris Habazettl for their excellent work and Dr. Hanns Ackermann (Institute for Biostatistics and Mathematic Modelling, Goethe University, Frankfurt am Main, Germany) for his kind help with the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Dr. Senckenbergische Stiftung, Frankfurt am Main.
Rights and permissions
About this article
Cite this article
Homola, M., Pfeffer, M., Robson, S.C. et al. Melatonin receptor deficiency decreases and temporally shifts ecto-5′-nucleotidase mRNA levels in mouse prosencephalon. Cell Tissue Res 365, 147–156 (2016). https://doi.org/10.1007/s00441-016-2378-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2378-x