Abstract
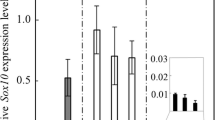
Some non-endocrine cells in the pituitary anterior lobe are responsible for providing stem/progenitor cells to maintain hormone-producing cells. In particular, cells expressing S100β protein, a calcium-binding protein, have been hypothesized to be a pituitary cell resource. Accumulating data have revealed that S100β-positive cells comprise heterogeneous populations and some of them certainly show stem/progenitor characteristics in vivo. Hence, we examine whether S100β-positive cells have the capacity to differentiate into endocrine cells, by means of in vivo and in vitro experiments on transgenic rats expressing enhanced green fluorescent protein (EGFP) under the control of the S100β promoter. Immunohistochemistry of the pituitary confirmed that some S100β-positive cells expressed SOX2 (SRY [sex-determining region Y]-box 2) and had proliferative activity. Dispersed anterior lobe cells were observed by time-lapse microscopy, followed by immunostaining for hormone and pituitary-transcription-factor1 (PIT1). First, the dispersed anterior lobe cells were immunostained by an antibody against SOX2. S100β-protein co-localizes with SOX2 (about 89 %). Although 44 of 134 S100β-positive cells traced were proliferative but negative to any hormones, 14 cells were positive for one of the pituitary hormones and/or PIT1, confirming the presence of all types of hormone-producing cells. Notably, GFP-fluorescence appeared in two hormone-positive cells during culture. On the other hand, we observed hormone-producing cells that were not positive for S100β at the end of the time-lapse study, despite being initially positive. These findings suggest that S100β-positive cells cultured from the anterior lobe are capable of developing into hormone-producing cells, although this happens relatively infrequently.






Similar content being viewed by others
References
Allaerts W, Vankelecom H (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol 153:1–12
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776
Brinkmeier ML, Davis SW, Carninci P, MacDonald JW, Kawai J, Ghosh D, Hayashizaki Y, Lyons RH, Camper SA (2009) Discovery of transcriptional regulators and signaling pathways in the developing pituitary gland by bioinformatic and genomic approaches. Genomics 93:449–460
Chen M, Kato T, Higuchi M, Yoshida S, Yako H, Kanno N, Kato Y (2013) Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary. Cell Tissue Res 354:823–836
Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, Hayashizaki Y, Arnhold IJ, Mendonca BB, Brue T, Camper SA (2010) Molecular mechanisms of pituitary organogenesis: in search of novel regulatory genes. Mol Cell Endocrinol 323:4–19
Devnath S, Inoue K (2008) An insight to pituitary folliculo-stellate cells. J Neuroendocrinol 20:687–691
Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912
Higuchi M, Kato T, Chen M, Yako H, Yoshida S, Kanno N, Kato Y (2013) Temporospatial gene expression of Prx1 and Prx2 is involved in morphogenesis of cranial placode-derived tissues through epithelio-mesenchymal interaction during rat embryogenesis. Cell Tissue Res 353:27–40
Horiguchi K, Fujiwara K, Kouki T, Kikuchi M, Yashiro T (2008) Immunohistochemistry of connexin 43 throughout anterior pituitary gland in a transgenic rat with green fluorescent protein-expressing folliculo-stellate cells. Anat Sci Int 83:256–260
Horiguchi K, Kikuchi M, Kusumoto K, Fujiwara K, Kouki T, Kawanishi K, Yashiro T (2010) Living-cell imaging of transgenic rat anterior pituitary cells in primary culture reveals novel characteristics of folliculo-stellate cells. J Endocrinol 204:115–123
Horiguchi K, Fujiwara K, Ilmiawati C, Kikuchi M, Tsukada T, Kouki T, Yashiro T (2011) Caveolin 3-mediated integrin beta1 signaling is required for the proliferation of folliculostellate cells in rat anterior pituitary gland under the influence of extracellular matrix. J Endocrinol 210:29–36
Horiguchi K, Fujiwara K, Yoshida S, Higuchi M, Tsukada T, Kanno N, Yashiro T, Tateno K, Osako S, Kato T, Kato Y (2014) Isolation of dendritic cell-like S100β-positive cells in rat anterior pituitary gland. Cell Tissue Res doi:10.1007/s00441-041-1817-9
Horiguchi K, Kouki T, Fujiwara K, Tsukada T, Ly F, Kikuchi M, Yashiro T (2012) Expression of the proteoglycan syndecan-4 and the mechanism by which it mediates stress fiber formation in folliculostellate cells in the rat anterior pituitary gland. J Endocrinol 214:199–206
Inoue K, Matsumoto H, Koyama C, Shibata K, Nakazato Y, Ito A (1992) Establishment of a folliculo-stellate-like cell line from a murine thyrotropic pituitary tumor. Endocrinology 131:3110–3116
Inoue K, Couch EF, Takano K, Ogawa S (1999) The structure and function of folliculo-stellate cells in the anterior pituitary gland. Arch Histol Cytol 62:205–218
Itakura E, Odaira K, Yokoyama K, Osuna M, Hara T, Inoue K (2007) Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology 148:1518–1523
Levy A (2002) Physiological implications of pituitary trophic activity. J Endocrinol 174:147–155
Levy A (2008) Stem cells, hormones and pituitary adenomas. J Neuroendocrinol 20:139–140
Mitsuishi H, Kato T, Chen M, Cai LY, Yako H, Higuchi M, Yoshida S, Kanno N, Ueharu H, Kato Y (2013) Characterization of a pituitary-tumor-derived cell line, TtT/GF, that expresses Hoechst efflux ABC transporter subfamily G2 and stem cell antigen 1. Cell Tissue Res 354:563–572
Nolan LA, Levy A (2006) A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J Neuroendocrinol 18:655–661
Nolan LA, Levy A (2009) The trophic effects of oestrogen on male rat anterior pituitary lactotrophs. J Neuroendocrinol 21:457–464
Nolan LA, Kavanagh E, Lightman SL, Levy A (1998) Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. J Neuroendocrinol 10:207–215
Nolan LA, Lunness HR, Lightman SL, Levy A (1999) The effects of age and spontaneous adenoma formation on trophic activity in the rat pituitary gland: a comparison with trophic activity in the human pituitary and in human pituitary adenomas. J Neuroendocrinol 11:393–401
Osuna M, Sonobe Y, Itakura E, Devnath S, Kato T, Kato Y, Inoue K (2012) Differentiation capacity of native pituitary folliculostellate cells and brain astrocytes. J Endocrinol 213:231–237
Rando TA (2006) Stem cells, ageing and the quest for immortality. Nature 441:1080–1086
Shirasawa N, Kihara H, Yamaguchi S, Yoshimura F (1983) Pituitary folliculo-stellate cells immunostained with S-100 protein antiserum in postnatal, castrated and thyroidectomized rats. Cell Tissue Res 231:235–249
Soji T, Yashiro T, Herbert DC (1989) Granulated “marginal cell layer” in the rat anterior pituitary gland. Tissue Cell 21:849–856
Susa T, Kato T, Yoshida S, Yako H, Higuchi M, Kato Y (2012) Paired-related homeodomain proteins Prx1 and Prx2 are expressed in embryonic pituitary stem/progenitor cells and may be involved in the early stage of pituitary differentiation. J Neuroendocrinol 24:1201–1212
Vankelecom H (2012) Pituitary stem cells drop their mask. Curr Stem Cell Res Ther 7:36–71
Vankelecom H, Chen J (2013) Pituitary stem cells: where do we stand? Mol Cell Endocrinol 385:2-17
Vankelecom H, Gremeaux L (2010) Stem cells in the pituitary gland: a burgeoning field. Gen Comp Endocrinol 166:478–488
Watkins-Chow DE, Camper SA (1998) How many homeobox genes does it take to make a pituitary gland? Trends Genet 14:284–290
Yako H, Kato T, Yoshida S, Inoue K, Kato Y (2011) Three-dimensional studies of Prop1-expressing cells in the rat pituitary primordium of Rathke’s pouch. Cell Tissue Res 346:339–346
Yako H, Kato T, Yoshida S, Higuchi M, Chen M, Kanno N, Cai L-Y, Hiroki U, Kato Y (2013) Three-dimensional studies of Prop1-expressing cells in the rat pituitary just before birth. Cell Tissue Res 354:837–847
Yoshida S, Kato T, Susa T, Cai L-Y, Nakayama M, Kato Y (2009) PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochem Biophys Res Commun 385:11–15
Yoshida S, Kato T, Yako H, Susa T, Cai L-Y, Osuna M, Inoue K, Kato Y (2011) Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol 23:933–943
Yoshida S, Kato T, Higuchi M, Yako H, Chen M, Kanno N, Ueharu H, Kato Y (2013) Rapid transition of NESTIN-expressing dividing cells from PROP1-positive to PIT1-positive advances prenatal pituitary development. J Neuroendocrinol 25:779–791
Yoshimura F, Soji T, Sato S, Yokoyama M (1977) Development and differentiation of rat pituitary follicular cells under normal and some experimental conditions with special reference to an interpretation of renewal cell system. Endocr J 24:435–449
Zhu X, Rosenfeld MG (2004) Transcriptional control of precursor proliferation in the early phases of pituitary development. Curr Opin Genet Dev 14:567–574
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963
Author information
Authors and Affiliations
Corresponding author
Additional information
Masashi Higuchi and Naoko Kanno contributed equally to this work.
This work was partially supported by JSPS KAKENHI (grant nos. 21380184 to Y.K. and 24580435 to T.K.) and by a research grant (A) to Y.K. from the Institute of Science and Technology, Meiji University. This study was also supported by the Meiji University International Institute for BioResource Research (MUIIR).
Rights and permissions
About this article
Cite this article
Higuchi, M., Kanno, N., Yoshida, S. et al. GFP-expressing S100β-positive cells of the rat anterior pituitary differentiate into hormone-producing cells. Cell Tissue Res 357, 767–779 (2014). https://doi.org/10.1007/s00441-014-1890-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1890-0