Abstract
Much of our current understanding of rare human diseases is driven by coding genetic variants. However, non-coding genetic variants play a pivotal role in numerous rare human diseases, resulting in diverse functional impacts ranging from altered gene regulation, splicing, and/or transcript stability. With the increasing use of genome sequencing in clinical practice, it is paramount to have a clear framework for understanding how non-coding genetic variants cause disease. To this end, we have synthesized the literature on hundreds of non-coding genetic variants that cause rare Mendelian conditions via the disruption of gene regulatory patterns and propose a functional classification system. Specifically, we have adapted the functional classification framework used for coding variants (i.e., loss-of-function, gain-of-function, and dominant-negative) to account for features unique to non-coding gene regulatory variants. We identify that non-coding gene regulatory variants can be split into three distinct categories by functional impact: (1) non-modular loss-of-expression (LOE) variants; (2) modular loss-of-expression (mLOE) variants; and (3) gain-of-ectopic-expression (GOE) variants. Whereas LOE variants have a direct corollary with coding loss-of-function variants, mLOE and GOE variants represent disease mechanisms that are largely unique to non-coding variants. These functional classifications aim to provide a unified terminology for categorizing the functional impact of non-coding variants that disrupt gene regulatory patterns in Mendelian conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genetic basis for multiple Mendelian conditions was initially identified by studying individuals harboring chromosomal translocations, which provided a signpost for where in the genome a gene was disrupted. It quickly became apparent that many of these chromosomal translocations did not disrupt coding sequence, but rather disrupted the positioning of coding sequence relative to a distal regulatory element or gene promoter (Vortkamp et al. 1991; Wallis et al. 1999; Fang et al. 2000; Crisponi et al. 2001). These initial studies helped establish that non-coding genetic variation can cause numerous Mendelian conditions, and work over the past several decades has solidified the central role of non-coding genetic variation in the pathogenesis of hundreds of Mendelian conditions.
In this review, we compiled hundreds of non-coding genetic variants from ClinVar and the literature that cause rare human diseases via the disruption of gene regulatory patterns. In doing so, we have recognized that there is no unified vocabulary for describing how this class of genetic variation contributes to Mendelian conditions. Specifically, as is detailed in the section “Functional categorization of gene regulatory variants”, the current functional classification system used for coding variants (i.e., loss-of-function, gain-of-function, and dominant-negative) is not well suited to non-coding variants, because it does not capture the diversity of functional consequences associated with this class of genetic variation. Furthermore, simply describing non-coding variants based on their distance to gene promoters is similarly inadequate. We present a new functional classification system for describing non-coding variants impacting regulatory elements (Table 1) and provide specific examples of variants that fall into each category of this new functional classification system (Tables 2, 3, 4).
Of note, non-coding variants can cause Mendelian conditions via a myriad of mechanisms, and this review specifically focuses on rare variants that cause Mendelian conditions by disrupting gene regulatory elements. Other classes of non-coding genetic variants include intronic variants that disrupt transcript splicing, 5’UTR genetic variants that alter initiation codon usage, 3’UTR and/or 5’UTR genetic variants that impact transcript stability, localization, or signal response, genetic variants within non-coding RNAs (ncRNAs), and non-coding repeat expansions that form altered RNA products (Stenson et al. 2017; French and Edwards 2020). Additionally, CpG methylation alterations at imprinted loci cause a handful of Mendelian conditions without altering the underlying DNA sequence (Cerrato et al. 2020). Common non-coding variants associated with common disease risk are covered elsewhere (Zhang and Lupski 2015; Spielmann and Mundlos 2016; French and Edwards 2020).
Structure of gene regulatory elements
Although the DNA content of a gene is present in every cell of the body, each gene may only be expressed within certain cell types and/or developmental time windows (herein referred to as the ‘intrinsic’ expression pattern of a gene). The intrinsic expression pattern of a gene is governed by regulatory elements, which are short DNA segments (often less than 400 bp in size) containing short binding elements (less than 30 bp) that govern the occupancy of sequence-specific transcription factors (TFs). Regulatory elements are distinguished based on their location relative to the transcriptional start site (TSS) of a gene (i.e., promoter-proximal, vs distal), as well as their functional impact on transcription (i.e., enhancers vs insulators vs silencers).
Promoters overlap the TSS of a gene and contain binding elements (e.g., TATA, CAAT, GC, CACCC boxes, etc.) that modulate RNA polymerase II binding and transcription (Juven-Gershon et al. 2008). Distal regulatory elements have complex roles in regulating the activity of promoters. For example, enhancers upregulate the transcriptional activity of a gene, and can be located adjacent to or over a megabase away from their target gene (Panigrahi and O’Malley 2021). Expression of each gene can be dependent on multiple enhancers, some of which may be common to several cell types. This combinatorial system allows different cell types to express the same gene through overlapping, but distinct, regulatory mechanisms. For example, there are nine different neural enhancers with overlapping spatial expression domains that drive Sonic Hedgehog (SHH) expression in the brain. Between different brain regions, the set of active enhancers is overlapping, but not identical. This mechanism allows for nuanced control of SHH expression in different parts of the brain (Amano 2020).
Distal regulatory elements can also serve as insulators, which function to compartmentalize adjacent gene regulatory domains along the genome (Gaszner and Felsenfeld 2006). For example, binding of the sequence-specific TF CTCF can create a barrier that limits an enhancer from regulating genes located on the opposite side of a CTCF boundary (Kim et al. 2015). CTCF-bound insulator sequences are often located at the boundaries of topologically associating domains (TADs), which are large chromatin loops (often > 100 kb in size) that enable the creation of transcriptionally independent chromatin domains wherein the activity of regulatory elements is primarily restricted to genes within the same TAD.
In addition, regulatory elements can exhibit context-specific activity. For example, some regulatory elements can act as either enhancers or silencers depending on their cellular context (Erceg et al. 2017; Huang and Ovcharenko 2022). Furthermore, the classification of regulatory elements is actively evolving as we learn how different regulatory elements influence gene expression in different cellular contexts, or in conjunction with neighboring regulatory elements (Ngan et al. 2020). Finally, although the overwhelming majority of variants that impact gene regulation are in the non-coding genome, coding variants can also impact gene regulatory elements (Lango Allen et al. 2014) as ~ 3% of all TF binding elements are located within coding sequences (Stergachis et al. 2013).
Functional categorization of gene regulatory variants
The functional consequence of coding variants is classified into three distinct categories, loss-of-function (LOF), gain-of-function (GOF), and dominant-negative (DN). LOF variants result in the loss of the normal biological function of a protein via either complete (amorphic) or partial (hypomorphic) LOF. In contrast, GOF variants create a protein with a function distinct from that of the wild-type protein via increasing protein activity (hypermorphic) or creating a completely new function (neomorphic). DN variants create a protein that either directly or indirectly blocks the normal function of the remaining wild-type protein (antimorphic). Notably, both GOF and DN variants are largely defined based on their alterations to the protein product of a gene. In contrast, since gene regulatory variants do not alter the protein sequence of a gene, but rather modulate gene expression patterns, these coding-centric categorizations are often ill suited for gene regulatory variants and create confusion.
We propose an alternative framework designed to functionally categorize genetic variants that disrupt gene regulatory elements. Specifically, we identify three distinct classes based on their impact on gene regulation at the level of gene transcripts (Table 1): (1) non-modular loss-of-expression (LOE) variants; (2) modular loss-of-expression (mLOE) variants; and (3) gain-of-ectopic-expression (GOE) variants. LOE variants are defined as variants that diminish or completely abolish the expression of a gene universally across all cell types that intrinsically express that gene. In contrast, mLOE variants are defined as variants that diminish or completely abolish the expression of a gene within a limited subset of the cell types or developmental windows that intrinsically express it (i.e., a modular loss of expression). GOE variants are defined as variants that result in the ectopic spatial and/or temporal expression of a gene (Fig. 1). Of note, unlike LOE variants, we chose not to further subdivide GOE variants into modular GOE variants, as it is quite challenging to obtain the appropriate clinical and molecular data that are necessary to firmly state that a GOE variant is truly limited to only a specific developmental window or cell type. For example, with mLOE variants, one can infer that there is a modular loss of expression based on a modular phenotype when compared to coding LOF variants in the same gene, which cause loss of protein function in all tissues or developmental windows. In contrast, with GOE variants, one cannot readily infer from clinical data that the ectopic gain of expression is limited to only a specific cell type. Specifically, a gene product may gain ectopic expression across all cell types, but only have a functional consequence in a select number of cell types—limiting the utility of tissue-selective phenotypes for inferring modular ectopic expression.
Schematic of the proposed gene regulatory variants functional classifications. a Schematic depicting the intrinsic expression pattern of a gene that is limited to the heart and brain, as well as the gene regulatory elements driving this intrinsic expression pattern. b–d Schematics depicting the impact of b LOE, c mLOE, and d GOE variants on the expression pattern of that gene across different tissues, as well as how various genetic variants can cause these changes
As opposed to the traditional LOF, GOF, and DN categories, this functional categorization more intuitively reflects the mechanisms by which disruptions in gene expression patterns cause Mendelian conditions. Of note, these functional classifications can be related to LOF or GOF variant types. For example, LOE variants can correspond to either amorphic or hypomorphic LOF variants. Although mLOE variants could be categorized as hypomorphic LOF variants, the mechanism by which mLOE variants cause disease is quite distinct from that of coding hypomorphic LOF variants, making ‘hypomorphic LOF’ an imprecise label for mLOE variants. In contrast, GOE variants can correspond to hypermorphic GOF, neomorphic GOF, and even LOF variants. Notably, there are no examples of DN gene regulatory variants causing Mendelian conditions in humans. However, this type of regulatory variant has been observed in other organisms and is termed “transvection” (Lewis 1954), which is a phenomenon where a regulatory element on one chromosome interacts with and enhances or silences its corresponding regulatory element on the homologous chromosome. More recently, this mechanism has been described in human cancers, wherein strong enhancers encoded on extrachromosomal circular DNA (ecDNA) can enhance the expression of autosomal genes (Zhu et al. 2021). It is possible that examples of transvection as a cause of Mendelian disease could be described in the future. The LOE, mLOE, and GOE functional categorizations represent the molecular consequences of regulatory variants more closely than the traditional LOF, DN, and GOF classification, and provide an improved framework for conceptualizing the putative role of novel gene regulatory variants in the pathogenesis of Mendelian conditions.
Non-modular loss-of-expression (LOE) variants
LOE variants diminish or abolish the expression of a gene across all cell types that intrinsically express that gene. Consequently, these variants often mirror the clinical manifestations of coding LOF variants for the same gene, as both LOE and LOF variants result in reduced/absent functional protein levels within the cell, albeit via distinct mechanisms. While some LOE variants cause complete loss of expression (analogous to amorphic LOF variants), others reduce the intrinsic expression level of a gene (analogous to hypomorphic LOF variants). The latter class of variants often results in a more attenuated clinical phenotype compared to variants that result in complete LOE. We have provided examples of over a hundred LOE variants (Table 2), and detail below some key examples of diverse LOE variants.
Many LOE variants are located within the gene promoter, where they disrupt essential TF binding elements required for the intrinsic expression of a gene. For example, genetic variants that disrupt the TATA box and/or CACCC box within the HBB gene promoter decrease the intrinsic expression of HBB by abrogating the ability of TFs to bind these elements. Notably, these variants do not completely abolish HBB transcription, and consequently, individuals harboring these variants in trans with HBB LOF variants often still produce adult hemoglobin (HbA), resulting in a milder form of beta-thalassemia (i.e., beta-thalassemia intermedia) compared to individuals with biallelic HBB LOF coding variants (Ropero et al. 2017).
Of note, different variants within the same gene promoter can cause varying magnitudes of LOE. For example, variants within the UROS promoter that disrupt the GATA1 or CP2-binding elements significantly reduce UROS transcription and cause a severe form of congenital erythropoietic porphyria (CEP), whereas other UROS promoter variants that do not disrupt these elements only cause a modest reduction in UROS transcription and mild cutaneous manifestations (Solis et al. 2001).
LOE variants can also disrupt distal regulatory elements. For example, monocytopenia and mycobacterial infection (MonoMAC) syndrome is typically caused by LOF coding variants within the gene GATA2. However, MonoMAC syndrome can also be caused by small deletions or single-nucleotide variants (SNVs) in a GATA2 intronic enhancer 9.5 kb downstream of the GATA2 promoter. These variants result in the loss of GATA2 expression via the disruption of enhancer TF binding elements that are essential for GATA2 transcription (i.e., an E-box, GATA, and ETS binding element) (Johnson et al. 2012; Hsu et al. 2013).
Variants within regulatory elements that are quite distal to a gene promoter can also cause LOE. For example, most cases of hereditary aniridia are caused by heterozygous LOF coding variants within PAX6. However, hereditary aniridia can also be caused by SNVs within a PAX6 enhancer located 150 kb downstream of the PAX6 promoter that disrupt a PAX6 autoregulatory element, causing loss of enhancer activity and subsequent loss of PAX6 transcription (Bhatia et al. 2013). Furthermore, some patients with hereditary aniridia will have deletions or chromosomal translocations that disrupt this PAX6 enhancer (Fantes et al. 1995), highlighting the diversity of genetic variant classes that can cause LOE.
Variants that disrupt insulators can also cause LOE. For example, a homozygous deletion of a CTCF-binding site within the first intron of LRBA has been reported to cause autoantibody-mediated pancytopenia, a phenotype associated with biallelic coding LOF LRBA variants (Turro et al. 2020). It is presumed that loss of this CTCF insulator element alters a TAD boundary, permitting heterochromatin spreading to silence LRBA promoter activity.
In summary, LOE variants can be located within promoter or distal gene regulatory elements, can completely mimic coding LOF variants or cause an attenuated phenotype relative to complete LOF variants, and are caused by diverse classes of genetic variants.
Modular loss-of-expression (mLOE) variants
In contrast to non-modular LOE variants, mLOE variants reduce or abolish the expression of a gene only in a subset of cell types that intrinsically express that gene. mLOE variants represent a disease mechanism largely unique to gene regulatory variants, as coding LOF variants typically disrupt the function of a gene across all cell types that intrinsically express that gene, with the exception of coding LOF variants within exons that are alternatively spliced only within certain tissues or somatic coding LOF variants that only exist within certain tissues (Poduri et al. 2013; Biesecker and Spinner 2013; Jaiswal and Ebert 2019). As a result of their modular impact on gene expression, mLOE variants can produce a subset of features associated with coding LOF variants in that same gene (i.e., phenotype modularity) (Table 3). As gene expression patterns are not typically measured across multiple tissues or developmental stages in individuals with Mendelian conditions, the modular nature of these variants is often inferred based on their phenotypic spectrum relative to individuals with coding LOF variants.
To illustrate the functional impact of mLOE variants, it is helpful to compare the full phenotype associated with coding LOF variants to the modular phenotype associated with mLOE variants in a gene regulatory element for the same gene. For example, coding LOF variants in GATA1 result in both severe platelet and red blood cell abnormalities, because GATA1 expression is critical for both of these cell types (Gutiérrez et al. 2020). In contrast, a 4 kb deletion of a megakaryocyte-specific enhancer element for GATA1 is associated with platelet abnormalities, but normal red blood cell parameters (Turro et al. 2020), as this enhancer is necessary for GATA1 expression within megakaryocytes but not within red blood cells.
Similarly, whereas coding LOF variants in PTF1A cause both pancreatic and cerebellar agenesis (Sellick et al. 2004), deletions or single-nucleotide variants within a pancreas-specific enhancer located 25 kb downstream of PTF1A cause only isolated pancreatic agenesis, likely because PTF1A expression during cerebellar neurogenesis is maintained (Weedon et al. 2014).
mLOE variants can also be located in promoters. For example, LOF variants in APC cause familial adenomatous polyposis, a condition associated with adenocarcinoma and numerous polyps in the stomach and colon. However, APC has two distinct promoters termed 1A and 1B, and APC transcription within the stomach mucosa is selectively initiated via promoter 1B. Consequently, individuals with variants in APC promoter 1B are at risk for developing gastric adenocarcinoma and proximal polyposis isolated to the stomach (GAPPS) without colon polyposis as a comorbidity (Li et al. 2016).
By selectively disrupting the expression of a gene in only a particular cell type, mLOE variants have the potential to produce a disease phenotype mediated by genes associated with embryonic lethality in the context of coding LOF variants. For example, biallelic LOF variants in PIGM are embryonic lethal in mice. In contrast, biallelic variants within the PIGM promoter that disrupt an SP1-binding element cause an inherited glycosylphosphatidylinositol deficiency characterized by a propensity for venous thrombosis and seizures (Almeida et al. 2006). The modular phenotype associated with this promoter variant results from the differential importance of this SP1 element in PIGM expression across cell types (Costa et al. 2014).
In addition to cell type selectivity, mLOE variants can also cause loss of expression at particular developmental stages. For example, variants that disrupt a C/EBP or HNF4-binding element within the F9 gene promoter cause Hemophilia B Leyden, which is characterized by severe factor IX deficiency at birth that ameliorates after puberty (Veltkamp et al. 1970). The affected C/EBP- and HNF4-binding elements are essential for F9 transcription in early childhood. However, after puberty, androgen-responsive TFs bind to an androgen response element within the F9 promoter, dramatically increasing F9 transcription to levels that largely resolve the disease phenotype (Crossley et al. 1992). Consequently, Hemophilia B Leyden is caused by a modular loss of F9 expression only within the prepubescent developmental stage.
In summary, mLOE variants are located within promoter or distal gene regulatory elements, can restrict the disease phenotype associated with coding LOF variants to only a specific tissue or developmental stage, and can result in a disease phenotype for genes wherein coding LOF variants would be embryonic lethal.
Gain-of-ectopic-expression (GOE) variants
GOE variants cause ectopic spatial and/or temporal expression patterns and represent a disease mechanism that is largely unique to regulatory variants (Table 4). Notably, some GOE variants can mimic Mendelian conditions caused by duplications of the target gene. For example, autosomal-dominant adult-onset demyelinating leukodystrophy (ADLD) is caused by overexpression of LMNB1 protein usually attributed to duplication of the LMNB1 gene. However, an ADLD family was discovered to have a deletion that begins 66 kb upstream of the LMNB1 promoter. This deletion encompasses a TAD boundary and results in overexpression of LMNB1 protein via a mechanism termed ‘enhancer adoption’. Specifically, a strong enhancer that typically does not regulate LMNB1 is now brought into the same TAD as the LMNB1 promoter, resulting in LMNB1 overexpression analogous to that seen with LMNB1 duplication (Giorgio et al. 2014).
Enhancer adoption is a common mechanism through which structural variants can cause regulatory element GOE (Fig. 1D). For example, structural variants within the WNT6/IHH/EPHA4/PAX3 locus can cause distinct phenotypes depending on where a strong cluster of limb enhancers for EPHA4 is situated relative to the WNT6, IHH, or PAX3 genes. Specifically, deletion of a TAD boundary between EPHA4 and PAX3 results in PAX3 adopting this cluster of limb enhancers, resulting in ectopic PAX3 expression and brachydactyly. In contrast, inversions or duplications involving IHH and the TAD boundary between IHH and EPHA4 result in WNT6 adopting this cluster of limb enhancers, resulting in ectopic WNT6 expression and F-syndrome (Lupiáñez et al. 2015).
SNVs within distal regulatory elements can also cause GOE. For example, the zone of polarizing activity regulatory sequence (ZRS), located in intron 5 of the LMBR1 gene, regulates SHH. SNVs within the ZRS located ~ 1 Mb upstream of SHH cause preaxial polydactyly (Lettice et al. 2002; Gurnett et al. 2007; Furniss et al. 2008) via the introduction of novel ETV2-binding sites in the ZRS, resulting in ectopic SHH expression within the developing limb bud (Koyano-Nakagawa et al. 2022). However, it is important to recognize that for a given gene, not all SNVs within distal regulatory elements result in the same phenotype, as non-coding SNVs within the SHH brain enhancer-2 (SBE2) located 460 kb upstream of SHH cause holoprosencephaly via an LOE mechanism (Jeong et al. 2008).
In addition to distal regulatory elements, GOE variants can also affect promoters. For example, glucocorticoid-remediable aldosteronism (GRA) is caused by ‘promoter switching’ between the genes CYP11B1 and CYP11B2, resulting in a chimeric gene wherein the adrenocorticotropic hormone (ACTH)-responsive promoter of the 11-beta-hydroxylase gene (CYP11B1) is fused with the coding region of the aldosterone synthase gene (CYP11B2) (Lifton et al. 1992). This results in ectopic expression of aldosterone synthase in zona fasciculata cells of the adrenal cortex, causing aldosterone synthase to be overexpressed and inducible by ACTH, hence a hyperaldosteronism state that normalizes upon treatment with glucocorticoids.
GOE variants can also cause Mendelian conditions for which the target gene does not have a known human phenotype associated with coding LOF variants, such as when coding LOF variants would result in embryonic lethality. This is notable, because the clinical identification of non-coding variants that cause Mendelian conditions is often informed by comparison to known LOF phenotypes. For example, complete loss of OVOL2 expression has been associated with embryonic lethality in mice, likely because OVOL2 is a transcription factor critical for epithelial cell lineage determination and differentiation (Mackay et al. 2006). Meanwhile, in humans, OVOL2 promoter variants that result in GOE can cause autosomal-dominant corneal endothelial dystrophies. These promoter variants result in the creation of binding elements for several activating TFs within the OVOL2 promoter, resulting in the inappropriate ectopic expression of OVOL2 in the developing or adult corneal endothelium (Davidson et al. 2016).
Promoter GOE variants can also disrupt the ability of transcriptional repressors to appropriately silence a gene at a particular developmental stage. For example, the gamma-globin genes HBG1 and HBG2 encode a component of fetal hemoglobin (HbF) and are normally expressed only during fetal erythropoiesis, as their promoters are silenced during adult erythropoiesis by the transcriptional repressors BCL11A and ZBTB7A. However, regulatory variants within the HBG1 and HBG2 promoters that disrupt BCL11A- and ZBTB7A-binding elements result in the hereditary persistence of fetal hemoglobin (HPFH) into adulthood (Martyn et al. 2018). As HbF is capable of preventing red blood cell sickling from sickle hemoglobin (HbS) and can compensate for deficient HbA as seen in beta-thalassemia, these HPFH variants can attenuate the phenotype of sickle cell disease and beta-thalassemia (Jackson et al. 1961; Cappellini et al. 1981; Labie et al. 1985; Weatherall 2001; Thein 2008; Thein et al. 2009). The discovery of HPFH variants has fortuitously enabled the development of gene editing therapies, which introduce these variants into adult erythroid progenitor cells to reactivate HbF as treatment for sickle cell disease and beta-thalassemia (Traxler et al. 2016; Li et al. 2021).
GOE variants and LOE variants impacting the same gene can lead to similar phenotypes. For example, a “Goldilocks” level of FOXG1 expression is likely required for normal brain development, because both FOXG1 duplications and deletions are associated with Rett-like phenotypes (Florian et al. 2012). Thus, it is unsurprising that GOE variants that remove a silencer and LOE variants that remove an enhancer have both been reported to cause Rett-like phenotypes via increasing and decreasing FOXG1 expression, respectively (Kortüm et al. 2011; Allou et al. 2012).
Finally, variants within gene regulatory elements can cause mixed effects. For example, the POMP gene typically has a short 5’UTR that originates from a TSS located at position c.-81. A single-nucleotide deletion in the POMP promoter at position c.-95 does not change the overall transcript levels of POMP, but results in decreased utilization of the canonical TSS and increased utilization of an upstream TSS located at position c.-181. This results in POMP transcripts that preferentially contain a long 5’UTR with reduced translational efficiency. Consequently, POMP expression within the granular layer of the epidermis is reduced, causing keratosis linearis with ichthyosis congenita and sclerosing keratoderma (KLICK) syndrome (Dahlqvist et al. 2010). This example illustrates how non-coding variants can have mixed effects, wherein they result in GOE of one transcript, LOE of a different transcript, and LOF at the protein level. In contrast, coding GOF variants in POMP result in proteasome-associated autoinflammatory syndrome 2 (PRAAS2) which has a quite distinct clinical presentation (Poli et al. 2018), demonstrating that gene regulatory GOE and coding GOF variants involving the same gene can cause completely different clinical phenotypes.
In summary, GOE can result from structural variants and SNVs located within promoters and distal gene regulatory elements. GOE variants often arise from the ectopic activity of enhancers (e.g., enhancer adoption) or promoters, or the disruption of normal repressive gene regulatory machinery. Furthermore, variants can cause complex gene regulatory outcomes wherein they cause GOE for one transcript, but LOE for a different one. Importantly, GOE variants often result in clinical phenotypes that markedly diverge from that of coding variants, complicating efforts to systematically identify this class of genetic variation using our current catalog of phenotypes associated with coding variants.
Concluding thoughts
In this review, we summarize the literature on gene regulatory variants that are known to cause Mendelian conditions and present a framework for categorizing these variants based on their proximate impact on gene expression patterns. We highlight that certain classes of gene regulatory variants can mimic coding LOF variants and gene duplication variants. However, gene regulatory variants can also create novel phenotypes. Specifically, the phenotypes associated with GOE and mLOE variants may markedly differ from those associated with LOE or LOF variants impacting the same gene. Consequently, extrapolating our knowledge of coding variants to the other 99% of the genome is insufficient for resolving how variants within gene regulatory elements cause Mendelian conditions. The current practice for identifying non-coding variants that cause Mendelian conditions often relies upon the phenotypic similarity to known coding LOF phenotypes, delaying or missing the identification of non-coding genetic variants when the resulting phenotype differs substantially from coding LOF of the same target gene. A functional classification system tailored to the impact of non-coding variants can facilitate the organization of knowledge, so that novel non-coding variants are more readily identified. Additionally, this functional classification system has the potential to improve how we integrate results from regulatory element mutational scanning experiments with observed genetic variants in databases like ClinVar. Specifically, this functional framework can serve as a standardized framework to articulate the functional impact of non-coding variants in relation to different disease phenotypes.
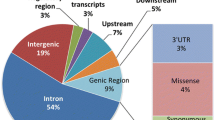
Although it has been well established for several decades that gene regulatory variants cause numerous Mendelian conditions in a dominant, recessive, or X-linked inheritance pattern, our current catalog of disease-causing variants is overwhelmingly populated with coding variants. Specifically, whereas ClinVar contains over 150,000 pathogenic or likely pathogenic coding variants (Landrum et al. 2018), our non-systematic review of the literature identified only several hundred genetic variants known to disrupt gene regulatory elements (Fig. 2). It is possible that this imbalance accurately reflects the relative contributions of coding and gene regulatory variants to Mendelian conditions. However, it is notable that the rate of discovery of non-coding regulatory variants has only modestly increased since the transition in 2010 from family-based linkage analysis to exome sequencing as the predominant mode for gene discovery and clinical testing (Fig. 2). In contrast, the rate of discovery of pathogenic coding variants has substantially increased over the past 10 years (Landrum et al. 2018; Bamshad et al. 2019). Consequently, we hypothesize the current imbalance in the identification of pathogenic coding variants over gene regulatory variants more likely reflects the inadequacy of exome sequencing and current tools for analysis and interpretation to implicate this class of variation in disease. As the use of genome sequencing and epigenetic profiling becomes more common within clinical genomics, we anticipate that more examples of gene regulatory variation causing Mendelian conditions will emerge.
Data availability
No new data were created or analyzed in this study.
References
Abicht A, Stucka R, Schmidt C et al (2002) A newly identified chromosomal microdeletion and an N-box mutation of the AChRε gene cause a congenital myasthenic syndrome. Brain 125:1005–1013. https://doi.org/10.1093/brain/awf095
Albers CA, Paul DS, Schulze H et al (2012) Compound inheritance of a low-frequency regulatory SNP and a rare null mutation in exon-junction complex subunit RBM8A causes TAR syndrome. Nat Genet 44:435–439. https://doi.org/10.1038/ng.1083
Allen HL, Caswell R, Xie W et al (2014) Next generation sequencing of chromosomal rearrangements in patients with split-hand/split-foot malformation provides evidence for DYNC1I1 exonic enhancers of DLX5/6 expression in humans. J Med Genet 51:264–267. https://doi.org/10.1136/jmedgenet-2013-102142
Allou L, Lambert L, Amsallem D et al (2012) 14q12 and severe Rett-like phenotypes: New clinical insights and physical mapping of FOXG1-regulatory elements. Eur J Hum Genet 20:1216–1223. https://doi.org/10.1038/EJHG.2012.127
Almeida AM, Murakami Y, Layton DM et al (2006) Hypomorphic promoter mutation in PIGM causes inherited glycosylphosphatidylinositol deficiency. Nat Med 12:846–851. https://doi.org/10.1038/nm1410
Amano T (2020) Gene regulatory landscape of the sonic hedgehog locus in embryonic development. Dev Growth Differ 62:334–342. https://doi.org/10.1111/dgd.12668
Bamshad MJ, Nickerson DA, Chong JX (2019) Mendelian Gene Discovery: Fast and Furious with No End in Sight. Am J Hum Genet 105:448–455. https://doi.org/10.1016/j.ajhg.2019.07.011
Benito-Sanz S, Thomas NS, Huber C et al (2005) A novel class of pseudoautosomal region 1 deletions downstream of SHOX is associated with Léri-Weill dyschondrosteosis. Am J Hum Genet 77:533–544. https://doi.org/10.1086/449313
Benito-Sanz S, Aza-Carmona M, Rodríguez-Estevez A et al (2012a) Identification of the first PAR1 deletion encompassing upstream SHOX enhancers in a family with idiopathic short stature. Eur J Hum Genet 20:125–127. https://doi.org/10.1038/ejhg.2011.210
Benito-Sanz S, Royo JL, Barroso E et al (2012b) Identification of the first recurrent PAR1 deletion in Léri-Weill dyschondrosteosis and idiopathic short stature reveals the presence of a novel SHOX enhancer. J Med Genet 49:442–450. https://doi.org/10.1136/jmedgenet-2011-100678
Benko S, Fantes JA, Amiel J et al (2009) Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nat Genet 41:359–364. https://doi.org/10.1038/ng.329
Berry M, Grosveld F, Dillon N (1992) A single point mutation is the cause of the Greek form of hereditary persistence of fetal haemoglobin. Nature 358:499–502. https://doi.org/10.1038/358499a0
Bhatia S, Bengani H, Fish M et al (2013) Disruption of autoregulatory feedback by a mutation in a remote, ultraconserved PAX6 enhancer causes aniridia. Am J Hum Genet 93:1126–1134. https://doi.org/10.1016/j.ajhg.2013.10.028
Biesecker LG, Spinner NB (2013) A genomic view of mosaicism and human disease. Nat Rev Genet 14:307–320. https://doi.org/10.1038/nrg3424
Borck G, Zarhrate M, Cluzeau C et al (2006) Father-to-daughter transmission of Cornelia de Lange syndrome caused by a mutation in the 5′ untranslated region of the NIPBL gene. Hum Mutat 27:731–735. https://doi.org/10.1002/humu.20380
Cai J, Goodman BK, Patel AS et al (2003) Increased risk for developmental delay in Saethre-Chotzen syndrome is associated with TWIST deletions: An improved strategy for TWIST mutation screening. Hum Genet 114:68–76. https://doi.org/10.1007/s00439-003-1012-7
Cappellini MD, Fiorelli G, Bernini LF (1981) Interaction between Homozygous β0 Thalassaemia and the Swiss Type of Hereditary Persistence of Fetal Haemoglobin. Br J Haematol 48:561–572. https://doi.org/10.1111/J.1365-2141.1981.TB02753.X
Cerrato F, Sparago A, Ariani F et al (2020) DNA Methylation in the Diagnosis of Monogenic Diseases. Genes (basel). https://doi.org/10.3390/genes11040355
Chen KJ, Chao HK, Hsiao KJ, Su TS (2002) Identification and characterization of a novel liver-specific enhancer of the human phenylalanine hydroxylase gene. Hum Genet 110:235–243. https://doi.org/10.1007/s00439-002-0677-7
Coffey AJ, Brooksbank RA, Brandau O et al (1998) Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet 20:129–135. https://doi.org/10.1038/2424
Collins FS, Boehm CD, Waber PG et al (1984) Concordance of a point mutation 5’ to the (G)γ globin gene with (G)γβ+ hereditary persistence of fetal hemoglobin in the black population. Blood 64:1292–1296. https://doi.org/10.1182/blood.v64.6.1292.1292
Costa FF, Zago MA, Cheng G et al (1990) The Brazilian type of nondeletional (A)γ-fetal hemoglobin has a C → G substitution at nucleotide -195 of the (A)γ-globin gene. Blood 76:1896–1897. https://doi.org/10.1182/blood.v76.9.1896.1896
Costa JR, Caputo VS, Makarona K et al (2014) Cell-type-specific transcriptional regulation of PIGM underpins the divergent hematologic phenotype in inherited GPl deficiency. Blood 124:3151–3154. https://doi.org/10.1182/blood-2014-09-598813
Cox JJ, Willatt L, Homfray T, Woods CG (2011) A SOX9 duplication and familial 46, XX developmental testicular disorder. N Engl J Med 364:91–93. https://doi.org/10.1056/NEJMc1010311
Craig JE, Sheerin SM, Barnetson R, Thein SL (1993) The molecular basis of HPFH in a British family identified by heteroduplex formation. Br J Haematol 84:106–110. https://doi.org/10.1111/j.1365-2141.1993.tb03032.x
Crisponi L, Deiana M, Loi A et al (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166. https://doi.org/10.1038/84781
Crossley M, Brownlee GG (1990) Disruption of a C/EBP-binding site in the factor IX promoter is associated with haemophilia B. Nature 345:444–446. https://doi.org/10.1038/345444a0
Crossley M, Winship PR, Austen DEG et al (1990) A less severe form of haemophilia B leyden. Nucleic Acids Res 18:4633. https://doi.org/10.1093/nar/18.15.4633
Crossley M, Ludwig M, Stowell KM et al (1992) Recovery from hemophilia B Leyden: an androgen responsive element in thefactor IX promoter. Science 257:377–379. https://doi.org/10.1126/science.1631558
Dahlqvist J, Klar J, Tiwari N et al (2010) A Single-Nucleotide Deletion in the POMP 5′ UTR Causes a Transcriptional Switch and Altered Epidermal Proteasome Distribution in KLICK Genodermatosis. Am J Hum Genet 86:596–603. https://doi.org/10.1016/J.AJHG.2010.02.018
Dathe K, Kjaer KW, Brehm A et al (2009) Duplications Involving a Conserved Regulatory Element Downstream of BMP2 Are Associated with Brachydactyly Type A2. Am J Hum Genet 84:483–492. https://doi.org/10.1016/j.ajhg.2009.03.001
Davidson AE, Liskova P, Evans CJ et al (2016) Autosomal-Dominant Corneal Endothelial Dystrophies CHED1 and PPCD1 Are Allelic Disorders Caused by Non-coding Mutations in the Promoter of OVOL2. Am J Hum Genet 98:75–89. https://doi.org/10.1016/j.ajhg.2015.11.018
De Angioletti M, Lacerra G, Gaudiano C et al (2002) Epidemiology of the delta globin alleles in southern Italy shows complex molecular, genetic, and phenotypic features. Hum Mutat 20:358–367. https://doi.org/10.1002/humu.10132
De Kok YJM, Vossenaar ER, Cremers CWRJ et al (1996) Identification of a hot spot for microdeletions in patients with X-linked deafness type 3 (DFN3) 900 kb proximal to the DFN3 gene POU3F4. Hum Mol Genet 5:1229–1235. https://doi.org/10.1093/hmg/5.9.1229
Dedoussis GVZ, Pitsavos C, Kelberman D et al (2003) FH-Pyrgos: A novel mutation in the promoter (-45delT) of the low-density lipoprotein receptor gene associated with familial hypercholesterolemia. Clin Genet 64:414–419. https://doi.org/10.1034/j.1399-0004.2003.00164.x
Delgado S, Velinov M (2015) 7q21.3 Deletion involving enhancer sequences within the gene DYNC1I1 presents with intellectual disability and split hand-split foot malformation with decreased penetrance. Mol Cytogenet. https://doi.org/10.1186/s13039-015-0139-2
Elsas LJ, Lai K, Saunders CJ, Langley SD (2001) Functional analysis of the human galactose-1-phosphate uridyltransferase promoter in Duarte and LA variant galactosemia. Mol Genet Metab 72:297–305. https://doi.org/10.1006/mgme.2001.3157
Erceg J, Pakozdi T, Marco-Ferreres R et al (2017) Dual functionality of cis-regulatory elements as developmental enhancers and Polycomb response elements. Genes Dev 31:590–602. https://doi.org/10.1101/gad.292870.116
Fakhouri WD, Rahimov F, Attanasio C et al (2014) An etiologic regulatory mutation in IRF6 with loss- and gain-of-function effects. Hum Mol Genet 23:2711–2720. https://doi.org/10.1093/hmg/ddt664
Fang J, Dagenais SL, Erickson RP et al (2000) Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet 67:1382–1388. https://doi.org/10.1086/316915
Fantes J, Redeker B, Breen M et al (1995) Aniridia-associated cytogenetic rearrangements suggest that a position effect may cause the mutant phenotype. Hum Mol Genet 4:415–422. https://doi.org/10.1093/hmg/4.3.415
Florian C, Bahi-Buisson N, Bienvenu T (2012) FOXG1-related disorders: From clinical description to molecular genetics. Mol Syndromol 2:153–163. https://doi.org/10.1159/000327329
Fonseca ACS, Bonaldi A, Bertola DR et al (2013) The clinical impact of chromosomal rearrangements with breakpoints upstream of the SOX9 gene: Two novel de novo balanced translocations associated with acampomelic campomelic dysplasia. BMC Med Genet. https://doi.org/10.1186/1471-2350-14-50
Foster JW, Dominguez-Steglich MA, Guioli S et al (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372:525–530. https://doi.org/10.1038/372525a0
French JD, Edwards SL (2020) The Role of Noncoding Variants in Heritable Disease. Trends Genet 36:880–891. https://doi.org/10.1016/j.tig.2020.07.004
Frischknecht H, Dutly F (2005) Two new δ-globin mutations: Hb A2-ninive [δ133(H11)Val→Ala] and a δ+-thalassemia mutation [-31 (A → G)] in the tata box of the δ-globin gene. Hemoglobin 29:151–154. https://doi.org/10.1081/HEM-200058593
Fucharoen S, Shimizu K, Fukumaki Y (1990) A novel C-T transition within the distal CCAAT motif of the Gγ-globin gene in the japanese HPFH: Implication of factor binding in elevated fetal globin expression. Nucleic Acids Res 18:5245–5253. https://doi.org/10.1093/nar/18.17.5245
Furniss D, Lettice LA, Taylor IB et al (2008) A variant in the sonic hedgehog regulatory sequence (ZRS) is associated with triphalangeal thumb and deregulates expression in the developing limb. Hum Mol Genet 17:2417–2423. https://doi.org/10.1093/hmg/ddn141
Galey M, Reed P, Wenger T et al (2022) 3-hour genome sequencing and targeted analysis to rapidly assess genetic risk. https://doi.org/10.1101/2022.09.09.22279746
Gallagher PG, Nilson DG, Wong C et al (2005) A dinucleotide deletion in the ankyrin promoter alters gene expression, transcription initiation and TFIID complex formation in hereditary spherocytosis. Hum Mol Genet 14:2501–2509. https://doi.org/10.1093/hmg/ddi254
Gaszner M, Felsenfeld G (2006) Insulators: Exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet 7:703–713. https://doi.org/10.1038/nrg1925
Gelinas R, Bender M, Lotshaw C et al (1986) Chinese (A)γ fetal hemoglobin: C to T substitution at position - 196 of the (A)γ gene promoter. Blood 67:1777–1779. https://doi.org/10.1182/blood.v67.6.1777.bloodjournal6761777
Giorgio E, Robyr D, Spielmann M et al (2014) A large genomic deletion leads to enhancer adoption by the lamin B1 gene: A second path to autosomal dominant adult-onset demyelinating leukodystrophy (ADLD). Hum Mol Genet 24:3143–3154. https://doi.org/10.1093/hmg/ddv065
Godart F, Bellanné-Chantelot C, Clauin S et al (2000) Identification of seven novel nucleotide variants in the hepatocyte nuclear factor-1α (TCF1) promoter region in MODY patients. Hum Mutat 15:173–180
Gotoh L, Inoue K, Helman G et al (2014) GJC2 promoter mutations causing Pelizaeus-Merzbacher-like disease. Mol Genet Metab 111:393–398. https://doi.org/10.1016/j.ymgme.2013.12.001
Gurnett CA, Bowcock AM, Dietz FR et al (2007) Two novel point mutations in the long-range SHH enhancer in three families with triphalangeal thumb and preaxial polydactyly. Am J Med Genet A 143:27–32. https://doi.org/10.1002/ajmg.a.31563
Gutiérrez L, Caballero N, Fernández-Calleja L et al (2020) Regulation of GATA1 levels in erythropoiesis. IUBMB Life 72:89–105. https://doi.org/10.1002/iub.2192
Hardison RC, Chui DHK, Giardine B et al (2002) HbVar. A relational database of human hemoglobin variants and thalassemia mutations at the globin gene server. Hum Mutat 19:225–233. https://doi.org/10.1002/humu.10044
Hatton CSR, Wilkie AOM, Drysdale HC et al (1990) α-Thalassemia caused by a large (62 kb) deletion upstream of the human α globin gene cluster. Blood 76:221–227. https://doi.org/10.1182/blood.v76.1.221.bloodjournal761221
Hazlewood RJ, Roos BR, Solivan-Timpe F et al (2015) Heterozygous triplication of upstream regulatory sequences leads to dysregulation of matrix metalloproteinase 19 in patients with cavitary optic disc anomaly. Hum Mutat 36:369–378. https://doi.org/10.1002/humu.22754
Henderson SJ, Timbs AT, McCarthy J et al (2016) Ten Years of Routine α - and β -Globin Gene Sequencing in UK Hemoglobinopathy Referrals Reveals 60 Novel Mutations. Hemoglobin 40:75–84. https://doi.org/10.3109/03630269.2015.1113990
Higgs DR, Wood WG (2008) Long-range regulation of α globin gene expression during erythropoiesis. Curr Opin Hematol 15:176–183. https://doi.org/10.1097/MOH.0b013e3282f734c4
Hill-Harfe KL, Kaplan L, Stalker HJ et al (2005) Fine mapping of chromosome 17 translocation breakpoints ≥900 Kb upstream of SOX9 in acampomelic campomelic dysplasia and a mild, familial skeletal dysplasia. Am J Hum Genet 76:663–671. https://doi.org/10.1086/429254
Hitchins MP, Rapkins RW, Kwok CT et al (2011) Dominantly Inherited Constitutional Epigenetic Silencing of MLH1 in a Cancer-Affected Family Is Linked to a Single Nucleotide Variant within the 5’UTR. Cancer Cell 20:200–213. https://doi.org/10.1016/j.ccr.2011.07.003
Horn S, Figl A, Rachakonda PS et al (2013) TERT promoter mutations in familial and sporadic melanoma. Sci 339:959–961. https://doi.org/10.1126/science.1230062
Houlden H, Girard M, Cockerell C et al (2004) Connexin 32 promoter P2 mutations: A mechanism of peripheral nerve dysfunction. Ann Neurol 56:730–734. https://doi.org/10.1002/ana.20267
Hsu AP, Johnson KD, Falcone EL et al (2013) GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood 121:3830–3837. https://doi.org/10.1182/blood-2012-08-452763
Huang D, Ovcharenko I (2022) Enhancer-silencer transitions in the human genome. Genome Res 32:437–448. https://doi.org/10.1101/gr.275992.121
Huang HJ, Stoming TA, Harris HF et al (1987) The greekaγβ+-hpfh observed in a large black family. Am J Hematol 25:401–408. https://doi.org/10.1002/ajh.2830250406
Huang L, Jolly LA, Willis-Owen S et al (2012) A noncoding, regulatory mutation implicates HCFC1 in nonsyndromic intellectual disability. Am J Hum Genet 91:694–702. https://doi.org/10.1016/j.ajhg.2012.08.011
Ilkovski B, Pagnamenta AT, O’Grady GL et al (2015) Mutations in PIGY: Expanding the phenotype of inherited glycosylphosphatidylinositol deficiencies. Hum Mol Genet 24:6146–6159. https://doi.org/10.1093/hmg/ddv331
Ionasescu VV, Searby C, Ionasescu R et al (1996) Mutations of the noncoding region of the connexin32 gene in X-linked dominant Charcot–Marie–Tooth neuropathy. Neurology 47:541–544. https://doi.org/10.1212/WNL.47.2.541
Jackson JF, Odom JL, Bell WN (1961) Amelioration of Sickle Cell Disease by Persistent Fetal Hemoglobin. JAMA J Am Med Assoc 177:867–869
Jaiswal S, Ebert BL (2019) Clonal hematopoiesis in human aging and disease. Science (1979). https://doi.org/10.1126/science.aan4673
Jakubiczka S, Schröder C, Ullmann R et al (2010) Translocation and deletion around SOX9 in a patient with acampomelic campomelic dysplasia and sex reversal. Sexual Development 4:143–149. https://doi.org/10.1159/000302403
Jamieson RV, Perveen R, Kerr B et al (2002) Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet 11:33–42. https://doi.org/10.1093/hmg/11.1.33
Jeong Y, Leskow FC, El-Jaick K et al (2008) Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet 40:1348–1353. https://doi.org/10.1038/ng.230
Johnson KD, Hsu AP, Ryu MJ et al (2012) Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J Clin Investig 122:3692–3704. https://doi.org/10.1172/JCI61623
Juven-Gershon T, Hsu JY, Theisen JW, Kadonaga JT (2008) The RNA polymerase II core promoter: the gateway to transcription. Curr Opin Cell Biol 20:253–259. https://doi.org/10.1016/j.ceb.2008.03.003
Kaneko K, Nagasaki Y, Furukawa T et al (2001) Analysis of the human pancreatic secretory trypsin inhibitor (PSTI) gene mutations in Japanese patients with chronic pancreatitis. J Hum Genet 46:293–297. https://doi.org/10.1007/s100380170082
Kim S, Yu NK, Kaang BK (2015) CTCF as a multifunctional protein in genome regulation and gene expression. Exp Mol Med. https://doi.org/10.1038/EMM.2015.33
Kioussis D, Vanin E, Delange T et al (1983) β-Globin gene inactivation by DNA translocation in γβ-thalassaemi. Nature 306:662–666. https://doi.org/10.1038/306662a0
Kortüm F, Das S, Flindt M et al (2011) The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J Med Genet 48:396–406. https://doi.org/10.1136/JMG.2010.087528
Koyano-Nakagawa N, Gong W, Das S et al (2022) Etv2 regulates enhancer chromatin status to initiate Shh expression in the limb bud. Nat Commun. https://doi.org/10.1038/s41467-022-31848-6
Kurth I, Klopocki E, Stricker S et al (2009) Duplications of noncoding elements 5′ of SOX9 are associated with brachydactyly-anonychia. Nat Genet 41:862–863. https://doi.org/10.1038/ng0809-862
Labie D, Pagnier J, Lapoumeroulie C et al (1985) Common haplotype dependency of high (G)γ-globin gene expression and high Hb F levels in β-thalasssemia and sickle cell anemia patients. Proc Natl Acad Sci U S A 82:2111–2114. https://doi.org/10.1073/PNAS.82.7.2111
Landrum MJ, Lee JM, Benson M et al (2018) ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res 46:D1062–D1067. https://doi.org/10.1093/NAR/GKX1153
Lango Allen H, Caswell R, Xie W et al (2014) Next generation sequencing of chromosomal rearrangements in patients with split-hand/split-foot malformation provides evidence for DYNC1I1 exonic enhancers of DLX5/6 expression in humans. J Med Genet 51:264–267. https://doi.org/10.1136/jmedgenet-2013-102142
Lecointre C, Pichon O, Hamel A et al (2009) Familial acampomelic form of campomelic dysplasia caused by a 960 kb deletion upstream of SOX9. Am J Med Genet A 149:1183–1189. https://doi.org/10.1002/ajmg.a.32830
Lee JA, Madrid RE, Sperle K et al (2006) Spastic paraplegia type 2 associated with axonal neuropathy and apparent PLP1 position effect. Ann Neurol 59:398–403. https://doi.org/10.1002/ana.20732
Leipoldt M, Erdel M, Bien-Willner GA et al (2007) Two novel translocation breakpoints upstream of SOX9 define borders of the proximal and distal breakpoint cluster region in campomelic dysplasia. Clin Genet 71:67–75. https://doi.org/10.1111/j.1399-0004.2007.00736.x
Lettice LA, Horikoshi T, Heaney SJH et al (2002) Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc Natl Acad Sci U S A 99:7548–7553. https://doi.org/10.1073/pnas.112212199
Lewis EB (1954) The theory and application of a new method of detecting chromosomal rearrangements in drosophila melanogaster. Am Nat 88:225–239. https://doi.org/10.1086/281833
Li J, Woods SL, Healey S et al (2016) Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am J Hum Genet 98:830–842. https://doi.org/10.1016/j.ajhg.2016.03.001
Li C, Georgakopoulou A, Mishra A et al (2021) In vivo HSPC gene therapy with base editors allows for efficient reactivation of fetal g-globin in b-YAC mice. Blood Adv 5:1122–1135. https://doi.org/10.1182/BLOODADVANCES.2020003702
Lifton RP, Dluhy RG, Powers M et al (1992) A chimaeric llβ-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355:262–265. https://doi.org/10.1038/355262a0
Lohan S, Spielmann M, Doelken SC et al (2014) Microduplications encompassing the sonic hedgehog limb enhancer ZRS are associated with haas-type polysyndactyly and Laurin–Sandrow syndrome. Clin Genet 86:318–325. https://doi.org/10.1111/cge.12352
Loudianos G, Lavinha PM, Galanello R et al (1992) Normal δglobin gene sequences in sardinian nondeletional δbeta;thalassemia. Hemoglobin 16:503–509. https://doi.org/10.3109/03630269208993118
Ludlow LB, Schick BP, Budarf ML et al (1996) Identification of a mutation in a GATA binding site of the platelet glycoprotein Ibβ promoter resulting in the Bernard–Soulier Syndrome. J Biol Chem 271:22076–22080. https://doi.org/10.1074/jbc.271.36.22076
Lupiáñez DG, Kraft K, Heinrich V et al (2015) Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161:1012–1025. https://doi.org/10.1016/j.cell.2015.04.004
Mackay DR, Hu M, Li B et al (2006) The mouse Ovol2 gene is required for cranial neural tube development. Dev Biol 291:38–52. https://doi.org/10.1016/j.ydbio.2005.12.003
Manco L, Ribeiro ML, Maximo V et al (2000) A new PKLR gene mutation in the R-type promoter region affects the gene transcription causing pyruvate kinase deficiency. Br J Haematol 110:993–997. https://doi.org/10.1046/j.1365-2141.2000.02283.x
Mandelker D, Zhang L, Kemel Y et al (2017) Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA J Am Med Assoc 318:825–835. https://doi.org/10.1001/jama.2017.11137
Mansour S, Hall CM, Pembrey ME, Young ID (1995) A clinical and genetic study of campomelic dysplasia. J Med Genet 32:415–420. https://doi.org/10.1136/jmg.32.6.415
Martyn GE, Wienert B, Yang L et al (2018) Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A-binding. Nat Genet 50:498–503. https://doi.org/10.1038/s41588-018-0085-0
Matsuda M, Sakamoto N, Fukumaki Y (1992) δ-Thalassemia caused by disruption of the site for an erythroid-specific transcription factor, GATA-1, in the δ-globin gene promoter. Blood 80:1347–1351. https://doi.org/10.1182/blood.v80.5.1347.1347
McElreavy K, Vilain E, Abbas N et al (1992) XY sex reversal associated with a deletion 5’ to the SRY “HMG box” in the testis-determining region. Proc Natl Acad Sci U S A 89:11016–11020. https://doi.org/10.1073/pnas.89.22.11016
Meeths M, Chiang SCC, Wood SM et al (2011) Familial hemophagocytic lymphohistiocytosis type 3 (FHL3) caused by deep intronic mutation and inversion in UNC13D. Blood 118:5783–5793. https://doi.org/10.1182/blood-2011-07-369090
Ngan CY, Wong CH, Tjong H et al (2020) Chromatin interaction analyses elucidate the roles of PRC2-bound silencers in mouse development. Nat Genet 52:264–272. https://doi.org/10.1038/s41588-020-0581-x
Ngcungcu T, Oti M, Sitek JC et al (2017) Duplicated Enhancer Region Increases Expression of CTSB and Segregates with Keratolytic Winter Erythema in South African and Norwegian Families. Am J Hum Genet 100:737–750. https://doi.org/10.1016/j.ajhg.2017.03.012
Nichols P, Croxen R, Vincent A et al (1999) Mutation of the acetylcholine receptor ε-subunit promoter in congenital myasthenic syndrome. Ann Neurol 45:439–443
Ninomiya S, Narahara K, Tsuji K et al (1995) Acampomelic campomelic syndrome and sex reversal associated with de novo t(12;17) translocation. Am J Med Genet 56:31–34. https://doi.org/10.1002/ajmg.1320560109
Ninomiya S, Isomura M, Narahara K et al (1996) Isolation of a testis-specific cDNA on chromosome 17q from a region adjacent to the breakpoint of t(12;17) observed in a patient with acampomelic campomelic dysplasia and sex reversal. Hum Mol Genet 5:69–72. https://doi.org/10.1093/hmg/5.1.69
Ohno K, Anlar B, Engel AG (1999) Congenital myasthenic syndrome caused by a mutation in the Ets-binding site of the promoter region of the acetylcholine receptor ε subunit gene. Neuromuscul Disord 9:131–135. https://doi.org/10.1016/S0960-8966(99)00007-3
Oner R, Kutlar F, Gu LH, Huisman THJ (1991) The Georgia type of nondeletional hereditary persistence of fetal hemoglobin has a C → T mutation at nucleotide -114 of the (A)γ-globin gene [1]. Blood 77:1124–1125. https://doi.org/10.1182/blood.v77.5.1124.1124
Otonkoski T, Jiao H, Kaminen-Ahola N et al (2007) Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic beta cells. Am J Hum Genet 81:467–474. https://doi.org/10.1086/520960
Panigrahi A, O’Malley BW (2021) Mechanisms of enhancer action: the known and the unknown. Genome Biol. https://doi.org/10.1186/s13059-021-02322-1
Pfeifer D, Kist R, Dewar K et al (1999) Campomelic dysplasia translocation breakpoints are scattered over 1 Mb proximal to SOX9: Evidence for an extended control region. Am J Hum Genet 65:111–124. https://doi.org/10.1086/302455
Pippucci T, Savoia A, Perrotta S et al (2011) Mutations in the 5′ UTR of ANKRD26, the ankirin repeat domain 26 gene, cause an autosomal-dominant form of inherited thrombocytopenia, THC2. Am J Hum Genet 88:115–120. https://doi.org/10.1016/j.ajhg.2010.12.006
Pirastu M, Galanello R, Doherty MA et al (1987) The same β-globin gene mutation is present on nine different β-thalassemia chromosomes in a Sardinian population. Proc Natl Acad Sci U S A 84:2882–2885. https://doi.org/10.1073/pnas.84.9.2882
Poduri A, Evrony GD, Cai X, Walsh CA (2013) Somatic mutation, genomic variation, and neurological disease. Science (1979). https://doi.org/10.1126/science.1237758
Poli MC, Ebstein F, Nicholas SK et al (2018) Heterozygous Truncating Variants in POMP Escape Nonsense-Mediated Decay and Cause a Unique Immune Dysregulatory Syndrome. Am J Hum Genet 102:1126–1142. https://doi.org/10.1016/j.ajhg.2018.04.010
Pop R, Conz C, Lindenberg KS et al (2004) Screening of the 1 Mb SOX9 5’ control region by array CGH identifies a large deletion in a case of campomelic dysplasia with XY sex reversal. J Med Genet. https://doi.org/10.1136/jmg.2003.013185
Refai O, Friedman A, Terry L et al (2010) De Novo 12;17 translocation upstream of sox9 resulting in 46, xx testicular disorder of sex development. Am J Med Genet A 152:422–426. https://doi.org/10.1002/ajmg.a.33201
Roessler E, Ward DE, Gaudenz K et al (1997) Cytogenetic rearrangements involving the loss of the Sonic Hedgehog gene at 7q36 cause holoprosencephaly. Hum Genet 100:172–181. https://doi.org/10.1007/s004390050486
Ropero P, Erquiaga S, Arrizabalaga B et al (2017) Phenotype of mutations in the promoter region of the β-globin gene. J Clin Pathol 70:874–878. https://doi.org/10.1136/jclinpath-2017-204378
Royle G, Van de Water NS, Berry E, et al (1991) Haemophilia B Leyden arising de novo by point mutation in the putative factor IX promoter region. Br J Haematol 77:191–194.
Sadiq MF, Eigel A, Horst J (2001) Spectrum of β-thalassemia in Jordan: Identification of two novel mutations. Am J Hematol 68:16–22. https://doi.org/10.1002/ajh.1143
Sakai T, Ohtani N, McGee TL et al (1991) Oncogenic germ-line mutations in Sp1 and ATF sites in the human retinoblastoma gene. Nature 353:83–86. https://doi.org/10.1038/353083a0
Schulert GS, Zhang M, Husami A et al (2018) Brief Report: Novel UNC13D Intronic Variant Disrupting an NF-κB Enhancer in a Patient With Recurrent Macrophage Activation Syndrome and Systemic Juvenile Idiopathic Arthritis. Arthritis and Rheumatology 70:963–970. https://doi.org/10.1002/art.40438
Schwarze U, Cundy T, Liu YJ et al (2019) Compound heterozygosity for a frameshift mutation and an upstream deletion that reduces expression of SERPINH1 in siblings with a moderate form of osteogenesis imperfecta. Am J Med Genet A 179:1466–1475. https://doi.org/10.1002/ajmg.a.61170
Sellick GS, Barker KT, Stolte-Dijkstra I et al (2004) Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet 36:1301–1305. https://doi.org/10.1038/ng1475
Senée V, Chelala C, Duchatelet S et al (2006) Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet 38:682–687. https://doi.org/10.1038/ng1802
Smemo S, Campos LC, Moskowitz IP et al (2012) Regulatory variation in a TBX5 enhancer leads to isolated congenital heart disease. Hum Mol Genet 21:3255–3263. https://doi.org/10.1093/hmg/dds165
Smyk M, Berg JS, Pursley A et al (2007) Male-to-female sex reversal associated with an ∼250 kb deletion upstream of NR0B1 (DAX1). Hum Genet 122:63–70. https://doi.org/10.1007/s00439-007-0373-8
Sobreira NLM, Gnanakkan V, Walsh M et al (2011) Characterization of complex chromosomal rearrangements by targeted capture and next-generation sequencing. Genome Res 21:1720–1727. https://doi.org/10.1101/gr.122986.111
Solis C, Aizencang GI, Astrin KH et al (2001) Uroporphyrinogen III synthase erythroid promoter mutations in adjacent GATA1 and CP2 elements cause congenital erythropoietic porphyria. J Clin Investig 107:753–762. https://doi.org/10.1172/JCI10642
Spielmann M, Mundlos S (2016) Looking beyond the genes: the role of non-coding variants in human disease. Hum Mol Genet 25:R157–R165. https://doi.org/10.1093/hmg/ddw205
Spielmann M, Brancati F, Krawitz PM et al (2012) Homeotic arm-to-leg transformation associated with genomic rearrangements at the PITX1 locus. Am J Hum Genet 91:629–635. https://doi.org/10.1016/j.ajhg.2012.08.014
Spitz F, Montavon T, Monso-Hinard C et al (2002) A t(2;8) balanced translocation with breakpoints near the human HOXD complex causes mesomelic dysplasia and vertebral defects. Genomics 79:493–498. https://doi.org/10.1006/geno.2002.6735
Stenson PD, Mort M, Ball EV et al (2017) The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet 136:665–677. https://doi.org/10.1007/s00439-017-1779-6
Stergachis AB, Haugen E, Shafer A et al (2013) Exonic transcription factor binding directs codon choice and affects proteinevolution. Sci 342:1367–1372. https://doi.org/10.1126/science.1243490
Su P, Ding H, Huang D et al (2011) A 4.6 kb genomic duplication on 20p12.2-12.3 is associated with brachydactyly type A2 in a Chinese family. J Med Genet 48:312–316. https://doi.org/10.1136/jmg.2010.084814
Sun M, Ma F, Zeng X et al (2008) Triphalangeal thumb-polysyndactyly syndrome and syndactyly type IV are caused by genomic duplications involving the long range, limb-specific SHH enhancer. J Med Genet 45:589–595. https://doi.org/10.1136/jmg.2008.057646
Tate VE, Wood WG, Weatherall DJ (1986) The British form of hereditary persistence of fetal hemoglobin results from a single base mutation adjacent to an S1 hypersensitive site 5’ to the (A)γ globin gene. Blood 68:1389–1393. https://doi.org/10.1182/blood.v68.6.1389.1389
Thein SL (2008) Genetic modifiers of the β-haemoglobinopathies. Br J Haematol 141:357–366. https://doi.org/10.1111/J.1365-2141.2008.07084.X
Thein SL, Menzel S, Lathrop M, Garner C (2009) Control of fetal hemoglobin: New insights emerging from genomics and clinical implications. Hum Mol Genet. https://doi.org/10.1093/HMG/DDP401
Tommerup N, Schempp W, Meinecke P et al (1993) Assignment of an autosomal sex reversa– locus (SRA1) and campomelic dysplasia (CMPD1) to 17q24.3–q25.1. Nat Genet 4:170–174. https://doi.org/10.1038/ng0693-170
Traxler EA, Yao Y, Wang Y-D et al (2016) A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat Med 22:987–990. https://doi.org/10.1038/nm.4170
Trembath DG, Semina EV, Jones DH et al (2004) Analysis of Two Translocation Breakpoints and Identifcation of a Negative Regulatory Element in Patients with Rieger’s Syndrome. Birth Defects Res A Clin Mol Teratol 70:82–91. https://doi.org/10.1002/bdra.10154
Turro E, Astle WJ, Megy K et al (2020) Whole-genome sequencing of patients with rare diseases in a national health system. Nature 583:96–102. https://doi.org/10.1038/s41586-020-2434-2
Van Wijk R, Van Solinge WW, Nerlov C et al (2003) Disruption of a novel regulatory element in the erythroid-specific promoter of the human PKLR gene causes severe pyruvate kinase deficiency. Blood 101:1596–1602. https://doi.org/10.1182/blood-2002-07-2321
Velagaleti GVN, Bien-Willner GA, Northup JK et al (2005) Position effects due to chromosome breakpoints that map ∼900 Kb upstream and ∼1.3 Mb downstream of SOX9 in two patients with campomelic dysplasia. Am J Hum Genet 76:652–662. https://doi.org/10.1086/429252
Veltkamp JJ, Meilof J, Remmelts HG et al (1970) Another Genetic Variant of Haemophilia B: Haemophilia B Leyden. Scand J Haematol 7:82–90. https://doi.org/10.1111/j.1600-0609.1970.tb01873.x
Vetro A, Ciccone R, Giorda R et al (2011) XX males SRY negative: A confirmed cause of infertility. J Med Genet 48:710–712. https://doi.org/10.1136/jmedgenet-2011-100036
Vinh DC, Patel SY, Uzel G et al (2010) Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 115:1519–1529. https://doi.org/10.1182/blood-2009-03-208629
Volkmann BA, Zinkevich NS, Mustonen A et al (2011) Potential novel mechanism for Axenfeld–Rieger syndrome: Deletion of a distant region containing regulatory elements of PITX2. Invest Ophthalmol vis Sci 52:1450–1459. https://doi.org/10.1167/iovs.10-6060
Vortkamp A, Gessler M, Grzeschik KH (1991) GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature 352:539–540. https://doi.org/10.1038/352539a0
Waber PG, Bender MA, Gelinas RE et al (1986) Concordance of a point mutation 5’ to the (A)γ-globin gene with (A)γβ+ hereditary persistence of fetal hemoglobin in Greeks. Blood 67:551–554. https://doi.org/10.1182/blood.v67.2.551.bloodjournal672551
Wagner T, Wirth J, Meyer J et al (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111–1120. https://doi.org/10.1016/0092-8674(94)90041-8
Wallis DE, Roessler E, Hehr U et al (1999) Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet 22:196–198. https://doi.org/10.1038/9718
Weatherall DJ (2001) Phenotype-genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat Rev Genet 2:245–255. https://doi.org/10.1038/35066048
Weedon MN, Cebola I, Patch A-M et al (2014) Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nat Genet 46:61–64. https://doi.org/10.1038/ng.2826
Wieczorek D, Pawlik B, Li Y et al (2010) A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner Mesomelic Syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum Mutat 31:81–89. https://doi.org/10.1002/humu.21142
Wieczorek D, Newman WG, Wieland T et al (2014) Compound Heterozygosity of Low-Frequency Promoter Deletions and Rare Loss-of-Function Mutations in TXNL4A Causes Burn–McKeown Syndrome. Am J Hum Genet 95:698–707. https://doi.org/10.1016/j.ajhg.2014.10.014
Wirth J, Wagner T, Meyer J et al (1996) Translocation breakpoints in three patients with campomelic dysplasia and autosomal sex reversal map more than 130 kb from SOX9. Hum Genet 97:186–193. https://doi.org/10.1007/BF02265263
Wu P, Zhang N, Wang X et al (2012) Family history of von Hippel-Lindau disease was uncommon in Chinese patients: Suggesting the higher frequency of de novo mutations in VHL gene in these patients. J Hum Genet 57:238–243. https://doi.org/10.1038/jhg.2012.10
Wunderle VM, Critcher R, Hastie N et al (1998) Deletion of long-range regulatory elements upstream of SOX9 causes campomelic dysplasia. Proc Natl Acad Sci U S A 95:10649–10654. https://doi.org/10.1073/pnas.95.18.10649
Yan H, Jin H, Xue G et al (2007) Germline hMSH2 promoter mutation in a Chinese HNPCC kindred: Evidence for dual role of LOH. Clin Genet 72:556–561. https://doi.org/10.1111/j.1399-0004.2007.00911.x
Young ID, Zuccollo JM, Maltby EL, Broderick NJ (1992) Campomelic dysplasia associated with a de novo 2q;17q reciprocal translocation. J Med Genet 29:251–252. https://doi.org/10.1136/jmg.29.4.251
Zertal-Zidani S, Merghoub T, Ducrocq R et al (1999) A novel C→A transversion within the distal CCAAT motif of the (G)γ- globin gene in the Algerian (G)γβ+-hereditary persistence of fetal hemoglobin. Hemoglobin 23:159–169. https://doi.org/10.3109/03630269908996160
Zhang F, Lupski JR (2015) Non-coding genetic variants in human disease. Hum Mol Genet 24:R102–R110. https://doi.org/10.1093/hmg/ddv259
Zhang F, Seeman P, Liu P et al (2010) Mechanisms for Nonrecurrent Genomic Rearrangements Associated with CMT1A or HNPP: Rare CNVs as a Cause for Missing Heritability. Am J Hum Genet 86:892–903. https://doi.org/10.1016/j.ajhg.2010.05.001
Zhu Y, Gujar AD, Wong C-H et al (2021) Oncogenic extrachromosomal DNA functions as mobile enhancers to globally amplify chromosomal transcription. Cancer Cell 39:694-707.e7. https://doi.org/10.1016/j.ccell.2021.03.006
Acknowledgements
The authors would like to thank Jessica Chong and Jacob Greene for their critical reading of this manuscript.
Funding
A.B.S. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and is a Pew Biomedical Scholar. This study was supported by National Institutes of Health (NIH) grants awarded to A.B.S. (1DP5OD029630 and OT2OD002748). S.C.B. was supported by a training grant (T32) from the NIH (T32GM007454). Y.H.H.C. is supported by NIGMS grant T32GM007266 (Univ. of WA Medical Scientist Training Program).
Author information
Authors and Affiliations
Contributions
All authors contributed to conceptualization of the manuscript. Y.H.H.C. and A.B.S. performed literature review. The first draft of the manuscript was written by A.B.S. and all authors provided critical revisions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, Y.H.H., Bohaczuk, S.C. & Stergachis, A.B. Functional categorization of gene regulatory variants that cause Mendelian conditions. Hum. Genet. 143, 559–605 (2024). https://doi.org/10.1007/s00439-023-02639-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-023-02639-w