Abstract
Giardia duodenalis is a common pathogenic intestinal protozoan parasite with high prevalence in developing countries, especially among children. The distribution of giardia assemblages among humans and their clinical relevance remains controversial. This study aimed to determine the prevalence and assemblage of Giardia among children under 5 years of age in Jimma, Southwest Ethiopia. Employing a case-control design, 606 children presenting with diarrhea at Jimma university medical center and Serbo Health Center were enrolled from December 2016 to July 2018 along with 617 matched controls without diarrhea. Giardia was detected and typed using real-time PCR. Univariate and multivariate regression analysis was performed. The total prevalence of Giardia was 41% (501/1223) and did not differ significantly between cases and controls (40% vs 42%). Prevalence increased by age, with the highest prevalence seen in children aged ≥ 25 months. Children without diarrhea with a history of diarrhea during the last month were more likely to be Giardia positive compared to children with no history diarrhea (OR 1.8 and 95%CI; 1.1–2.9). Regardless of current diarrhea symptoms, assemblage B predominated with 89%, followed by assemblage A (8%) and mixed infection assemblage A and B (3%). We report a high prevalence of Giardia by PCR detection in Jimma, Ethiopia, with assemblage B being predominant. There was a similar distribution of Giardia assemblages between children with and without diarrhea. Increasing age was a risk factor for Giardia infection. Community-based prevention and control strategies need to be employed to decrease the risk of giardia infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giardia duodenalis (syn. Giardia lamblia and Giardia intestinalis) causes Giardia infection in humans and many mammals. It is transmitted through the oral-fecal route following direct or indirect contact with the infectious cyst stages, including human-to-human, zoonotic, waterborne, and foodborne transmission (Einarsson et al. 2016; Feng and Xiao 2011).
The spectrum of clinical symptoms that occur in infected individuals may range from asymptomatic to acute or chronic diarrheal disease. When present, the clinical signs may include diarrhea, nausea, weight loss, bloating, and abdominal pain (Adam 2021). Furthermore, chronic infection can result in stunting and reduced psychomotor development (Rogawski et al. 2017; Kabir et al. 2022). Giardiasis can also cause severe malabsorption resulting in malabsorption of fat, proteins, folic acid, vitamin A, and vitamin B12 (Kabir et al. 2022; Keselman et al. 2016; Al-Mekhlafi et al. 2010).
Giardiasis is especially common in areas with poor sanitation and no or insufficient water treatment facilities (Leder and Weller 2020). The prevalence of giardiasis ranges from 3 to 7% in developed countries and 20 to 30% in developing countries (Leung et al. 2019; Mahdavi et al. 2022) and even more than 30% in Ethiopia (de Lucio et al. 2016; Gelanew et al. 2007). The variation in prevalence might be attributed to factors such as the geographical area, urban or rural settings, age group composition, hygienic standards, and the socio-economic conditions of the study subjects.
Molecular studies reveal eight distinct genetic assemblages of Giardia duodenalis, i.e., assemblages A to H. Two of the assemblages, A and B, are widely known to infect humans and other mammals (Heyworth 2016). The remaining six assemblages (C to H) are host-specific and infect animals, even though assemblages C (Soliman et al. 2011), E (Fantinatti et al. 2016), and F (Gelanew et al. 2007; Pipikova et al. 2020) have been reported from human isolates. There is extensive geographical variation in G. duodenalis assemblages within countries and between different countries and continents (Hijjawi et al. 2022). For example within Ethiopia, some reported higher rate of assemblage B (Tigabu et al. 2010; Wegayehu et al. 2013; Birrie and Erko 2017) while other studies reported higher prevalence of assemblage A (Gelanew et al. 2007; Hajare et al. 2022) using different types of PCR techniques targeting glutamate dehydrogenase, beta-giardin, and/or triosephosphate isomerase genes or sequencing.
There are discrepancies in the reported Giardia prevalence within Ethiopia. Most available reports on prevalence of G. duodenalis infection in Ethiopia have used conventional microscopy for detection of Giardia. These studies in North Shoa, Benishangul Gumuz, Sidama, Lege Dini, Eastern Ethiopia, and Jimma town reported prevalence ranging from 5 to 35% (Ayalew et al. 2008; Belete et al. 2021; Beyene and Tasew 2014; Damitie et al. 2018; Flecha et al. 2015; Kifleyohannes et al. 2022; Mengistu et al. 2007; Wegayehu et al. 2016). Some studies used PCR for detection of Giardia and found prevalence ranging from 11 to 55% in Southern Oromia, central Ethiopia, Southern Ethiopia, Tigrai North Ethiopia, and in Northwest Amhara regions (de Lucio et al. 2016; Tigabu et al. 2010; Wegayehu et al. 2013; Birrie and Erko 2017; Hajare et al. 2022).
Studies on the molecular epidemiology of G. duodenalis from different parts of the world help to clarify possible relationships among genetic diversity of the parasite, clinical presentation, and environmental transmission dynamics (Feng and Xiao 2011; Cacciò and Sprong 2011). So far, there are no studies using PCR to detect and characterize Giardia in the larger Jimma area in Ethiopia. Therefore, using a case-control design, we aimed to determine the prevalence, molecular epidemiology, and risk factors of Giardia infection among under 5-year-old children in Jimma, Southwest Ethiopia.
Methods and materials
Study area
The study was conducted in Jimma town (Jimma University Medical Centre, JUMC), located 346 km to the southwest of the capital Addis Ababa, and Kersa woreda (Serbo health center) located 18 km to the east of Jimma. Jimma town has a total population of 120,960 and is the administrative center for 21 surrounding districts. Jimma is largely coffee producing agrarian community.
Study design and period
This study was part of a larger prospective case-control study, which investigated diagnostic accuracy of LED-AP for cryptosporidiosis (Johansen et al. 2021), conducted from December 2016 to July 2018.
Study participant
All children under 5 years of age presenting with diarrhea at JUMC and Serbo Health Center were included after obtaining parents’ or guardians’ written informed consent. The study enrolled children younger than 5 years who presented with diarrhea (three or more loose stools within the previous 24 h), or dysentery (at least one loose stool with stains of blood within the previous 24 h). The study included cases with prolonged (7–13 days) and persistent (≥14 days) diarrhea. Community controls without diarrhea in the preceding 48 h were enrolled concurrently by weekly recruitment plans using frequency matching to cases by sex, age stratum, and geographical location of households.
Data collection and laboratory procedures
Demographic and clinical data were collected by study nurses using standardized case report forms by interview and from hospital records. All parents/caretakers of the participants were instructed and asked to provide a stool sample of their children with a screw-cap plastic container. Aliquots of stool samples were stored at −80°C until shipment to Vestfold Hospital Trust, Norway, for further processing. During the time the study was conducted a wet microscopy was performed as soon as the stool sample reached the laboratory. A result slip with the wet microscopy findings was immediately brought back to the treating health care worker (doctor or nurse). The presence or absence of Giardia cysts or trophozoites were reported on this result slip. The decision to treat was left with the treating clinical team who followed local standard treatment regimen.
Total nucleic acid extraction was performed on aliquots of stool samples. Briefly, a thawed aliquot of stool was added to (pre-made) 500 μl S.T.A.R. buffer (Roche) + 500 μl BLB (MagNA Pure bacterial lysis buffer, Roche) and vortexed, and the stool-buffer suspension was kept frozen. Stool-buffer suspension was then used for nucleic acid extraction with MagNA Pure 96 instrument, MagNA Pure 96 DNA, and Viral NA Large Volume Kit, and eluted in 100 μl. Giardia was detected using real-time qPCR run on a Light Cycler 480II, Roche, including primers and probes for the internal control (DNA process control kit, Roche) (Johansen et al. 2021).
All G. duodenalis positive samples were further analyzed using an assemblage-specific real-time PCR assay (Van Lith et al. 2015). Primers targeting assemblage-specific genes: Translation Initiation Factor (locus GL50803_39587) and Cathepsin L precursor (locus GL50581_3714) were used as markers for assemblages A and B, respectively using a Light Cycler® 480 instrument. Each real-time PCR reaction consisted of 2×LightCycler 480 SYBR Green I Master mix, 3 pmoles of each primer, 2 μl of genomic DNA, and sterile water up to a final volume of 20 μl. Experimental conditions consisted of 5 min incubation at 95 °C, followed by 45 cycles of denaturation at 95 °C for 12 s, annealing at 55 °C for 12 s, and extension at 72 °C for 12 s. Fluorescence data were collected as a single acquisition at the end of each cycle. The melting curve analysis was performed at the end of each reaction and consisted of 95 °C for 5 s, 60 °C for 1 min, and heating to 97 °C with continuous acquisitions. Negative controls (water instead of DNA) as well as DNA from G. duodenalis assemblage A WB Clone C6 ATCC strain and an assemblage B cyst isolate, verified by tpi sequencing were included in each run. Cycle threshold (CT) values and melting curve values were recorded.
Statistical analysis
The statistical analysis was carried out using the SPSS® statistics program, version 20. Univariate and multivariate regression models were used to assess possible risk factors for acquiring Giardia. Results were interpreted using odds ratios, 95% confidence intervals, and significance levels. For analysis of acute malnutrition, children < 6 months, severe acute malnutrition (SAM) was defined as weight for-height z-score (WHZ) ≤ −3 of the WHO standard curves (WHO Multicentre Growth Reference Study Group 2006) and/or presence of bilateral edema involving at least the feet. Moderate acute malnutrition (MAM) was defined as a WHZ ≤ −2 and > −3 with no edema. Mid-upper arm circumference (MUAC) was used instead of WHZ for 6- to 59-month-olds, as it was difficult to bring height measurement boards to community-control home visits (usually done by motorcycle), and because MUAC is less susceptible to dehydration than weight (Modi et al. 2015; Mwangome et al. 2011); SAM was defined as MUAC ≤ 115 mm and/or presence of bilateral edema involving at least the feet, and MAM was defined a MUAC >115 mm and ≤ 125 mm with no edema. During analysis Acute malnutrition combined SAM and MAM.
Results
The study population
The study included 1223 children of which 674 (55%) were males and 549 (45%) were females. Out of 1223 children, 58% and 42% were from Serbo and Jimma study sites respectively. The most common age category was 7–12 months (33%), followed by the 13–24 months (32%). Using anthropometric measurements most children (88%) had no acute malnutrition, 51% kept animals in the house and 98% of children with diarrhea had acute diarrhea lasting less than 14 days (Table 1).
Prevalence and quantity of G. duodenalis among the cases and controls study participants
Of the 1223 study participants, 606 and 617 were cases and controls, respectively. The prevalence of G. duodenalis was 40% (240/606) in cases and 42% (261/617) in controls. The prevalence of Giardia in participants of this study was not significantly different among cases and controls OR 1.1, 95% CI (0.9–1.2). Because history of diarrhea in the last one month was a risk for detecting G. duodenalis in stool, we did sensitivity analysis by excluding children with history of diarrhea in the past month. The prevalence of Giardia was quite similar in cases and controls 41%, 212/522 vs. 38%, 193/506 OR 1.12, 95% CI (0.9–1.4). We further assessed the quantity of G. duodenalis in the samples based on the Cq values obtained in qPCR using non-parametric test. We found a higher quantity of Giardia among children without diarrhea (mean Cq value 26.6) compared to children with diarrhea (mean Cq value 27.5) (P=0.006).
Association between Giardia and different demographic and clinical characteristics
Tables 2 and 3 show the association between G. duodenalis and different demographic and clinical characteristics in univariate and multivariate analysis, respectively. The prevalence of G. duodenalis in study participants was increasing with age, the highest prevalence was seen among children ≥ 25 months in both cases and controls, OR 23.75, 95% CI (10.31–54.73) P = <0.001. Children without diarrhea, with a history of diarrhea in the past month had increased risk of having a Giardia positive stool sample (OR 1.8; 95%CI 1.1–2.9). Other demographic or clinical characteristics such as sex, nutritional status, domestic animals in the house, type of diarrhea, and vomiting were not associated with Giardia infection.
G. duodenalis typing results
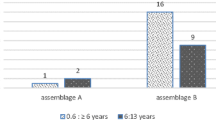
Out of a total of 501 G. duodenalis PCR positive samples, 281 (56%) samples could be typed of which 132 and 149 samples were from children with diarrhea and children without diarrhea respectively. Assemblage B was found in 89% of samples followed by assemblage A (8%) and 3% was a mixed infection of assemblage A+B. Chi-square test was used to compare the differences in proportion of Giardia assemblage types in cases and control; results in Table 4 show that there were no significant differences (P=0.48) of the assemblage types between cases and control samples. We further assessed distribution of assemblages in two different age groups i.e., children aged <24 months (n=165) and children above 24 months (n=116). Out of 165 samples from children aged <24 months, 147 (89%) had assemblage B, while 15 (9%) and 3 (2%) had assemblage A and mixed assemblage A+B respectively. From 116 samples of children aged >24 months, 104 samples (90%) had assemblage B, 7 (6%) had assemblage A, and 5 (4%) had mixed assemblage A+B. There were no significant age differences in G. duodenalis assemblage distributions.
Discussion
This study is one of the few studies assessing the prevalence, assemblage, and risk factors of Giardia infection among pediatric population in Jimma Ethiopia. Additionally, to the best of our knowledge, this is one of the first studies to use assemblage-specific PCR and employing a case-control design in children under 5 years in Ethiopia.
Based on diagnostic real-time PCR, the overall prevalence of G. duodenalis among participants in this case-control study was 40%. In earlier studies within the country, some indicated a lower prevalence of 10–18% (Birrie and Erko 2017; Hajare et al. 2022; Wegayehu et al. 2016) while one study in North-west Ethiopia reported higher prevalence of 55% (de Lucio et al. 2016) than the current study. Comparing our findings with different African countries, there are differences in prevalence reported where some countries reported low G. duodenalis prevalence; Kenya 4.5% (Mbae et al. 2016), Tanzania 4.6% (Tellevik et al. 2015), Ghana 5.6% (Anim-Baidoo et al. 2016), Egypt 24.4% (Ahmad et al. 2020), and Mozambique 27.4% (Messa Jr et al. 2021). Other countries such as Rwanda (Ignatius et al. 2012) and Uganda (Al-Shehri et al. 2019) reported high Giardia prevalence of 60.1% and 87%, respectively. The marked variation in G. duodenalis prevalence is likely related to the varying sensitivity of the diagnostic methods used across studies. Studies which used PCR, generally considered a more sensitive method (Stensvold and Nielsen 2012), tend to report higher prevalence (de Lucio et al. 2016; Ignatius et al. 2012; Al-Shehri et al. 2019) compared to studies using a conventional microscopy (Mbae et al. 2016; Ahmad et al. 2020; Messa Jr et al. 2021). Secondly, the age of the study population matters, as shown in this study G. duodenalis detection increased with age. Studies which included older children like Rwanda (Ignatius et al. 2012) and other parts of Ethiopia (de Lucio et al. 2016) reported high G. duodenalis prevalence. Thirdly, geographical variation and socio-economic factors, such as access to safe drinking water, sanitation, and hygiene practices, may also be the cause of variation in G. duodenalis prevalence (Nundy et al. 2011). Some studies, e.g., in Tanzania (Tellevik et al. 2015) and Ethiopia (Wegayehu et al. 2016) reported low prevalence of G. duodenalis of 4.6% and 16.8% despite using PCR.
We found high prevalence of G. duodenalis among participants in this case-control study without significant differences even after removing from the analysis children with a history of diarrhea in the past month. Children without diarrhea with G. duodenalis may serve as parasite reservoirs. Several studies in developing countries reported higher prevalence of G. duodenalis in controls than in cases with acute diarrhea (Tellevik et al. 2015; Anim-Baidoo et al. 2016; Ahmad et al. 2020; Messa Jr et al. 2021; Ignatius et al. 2012; Al-Shehri et al. 2019; Becker et al. 2015; Muhsen and Levine 2012) and the parasite is overlooked in from global burden estimations of diarrheal diseases (GBD 2013 Mortality and Causes of Death Collaborators 2015). The frequent detection of Giardia in both case and control groups serves as a compelling indication that waterborne pathogens are commonly transmitted among the studied age groups. Consequently, future research endeavors should explore detection of additional pathogens such as bacterial and viruses to precisely identify the specific etiological factors contributing to diarrhea within this study cohort.
G. duodenalis infection was significantly associated with increasing age. Age-related increase in prevalence of infection has also been observed in other studies (Mbae et al. 2016; Tellevik et al. 2015; Ignatius et al. 2012). Children without diarrhea with history of diarrhea for the last one month before the study had increased risk of Giardia detection from stool. This could be due to prolonged shedding of G. duodenalis cyst in stool from the previous diarrhea episode or asymptomatic giardiasis. No other demographic or clinical characteristic assessed in our study such as sex, acute malnutrition, and keeping animals in the house were associated with G. duodenalis.
One of the limitations of the present study was that only 50% of the samples could be typed. Based on the assemblage-specific real-time PCR employed, G. duodenalis assemblage B was the predominant subtype with a prevalence of 89%. Similarly, assemblage B was reported in higher frequency than assemblage A in previous studies conducted in Ethiopia (de Lucio et al. 2016; Wegayehu et al. 2016) and other African countries (Tellevik et al. 2015; Messa Jr et al. 2021; Ignatius et al. 2012; Al-Shehri et al. 2019). On the other hand, assemblage A was also reported higher in some other studies in Ethiopia and other countries (Gelanew et al. 2007; Hajare et al. 2022; Ahmad et al. 2020). The variation in the predominance of G. duodenalis assemblage may partly be explained by either the genetic diversity of G. duodenalis circulating in different geographical areas or the difference in the dynamics of transmission. There are controversial reports on the role of G. duodenalis assemblages and clinical symptoms (Haque et al. 2009; Haque et al. 2005; Molina et al. 2011; Read et al. 2002). In this study, we did not find significant differences in G. duodenalis assemblages between children with and without diarrhea. However, studies from Rwanda, Australia, and Bangladesh have shown that G. duodenalis assemblage A was more likely to be found in symptomatic diarrheic children (Ignatius et al. 2012; Haque et al. 2009; Haque et al. 2005; Read et al. 2002), while in Argentina children presented with abdominal pain were more likely to be found with G. duodenalis assemblage B (Molina et al. 2011). Further molecular studies are needed to clarify the association between G. duodenalis assemblage and diarrhea symptoms. In the present study mixed infection of G. duodenalis assemblage A and B was found in 3 percent of cases and has been reported previously in Ethiopia and other countries (Hijjawi et al. 2022). The occurrence of mixed infections by several assemblages of G. duodenalis suggests the intricate circulation of the parasite within the environment and study participants may have been exposed to multiple sources of infection (Tamura et al. 2011). To comprehensively understand this, further studies are needed to characterize the origins of contamination and gain insight into varying contributions of anthroponotic, zoonotic, and environmental modes of transmission. Furthermore, we did not find any correlation between G. duodenalis assemblages and other clinical or socio-demographic parameters. This could be due to the strong dominance of assemblage B in our sample set which resulted in low power to assess correlations.
Higher risk of G. duodenalis in rural areas, has also been reported in other studies (Samie et al. 2020) and is likely caused by individuals, especially children, being in closer contact with natural sources of water and soil, thereby increasing risk of transmission (Addy et al. 2004). Increased in G. duodenalis infection prevalence during the rainy season was observed in studies conducted in Zambia (Siwila et al. 2011), Southwest London (Breathnach et al. 2010), and Canada (Brunn et al. 2019). In the present study, no clear seasonal pattern emerged.
Conclusion
Using PCR method, we report a prevalence of 41% of G. duodenalis in this study from Jimma Ethiopia. Assemblage B was the predominant type causing giardiasis in this region. Giardia prevalence increased with age. The occurrence of diarrhea symptoms was not associated with G. duodenalis infection, nor with Giardia assemblage.
Data availability
All data generated or analysed during this study are included in this published article.
References
Adam RD (2021) Giardia duodenalis: biology and pathogenesis. Clin Microbiol Rev 34(4):e0002419
Addy PA, Antepim G, Frimpong EH (2004) Prevalence of pathogenic Escherichia coli and parasites in infants with diarrhoea in Kumasi. Ghana East Afr Med J 81(7):353–357
Ahmad AA, El-Kady AM, Hassan TM (2020) Genotyping of Giardia duodenalis in children in upper Egypt using assemblage- specific PCR technique. PLoS One 15(10):e0240119
Al-Mekhlafi HM et al (2010) Giardiasis and poor vitamin A status among aboriginal school children in rural Malaysia. Am J Trop Med Hyg 83(3):523–527
Al-Shehri H et al (2019) Molecular characterisation and taxon assemblage typing of giardiasis in primary school children living close to the shoreline of Lake Albert Uganda. Parasite Epidemiol Control 4:e00074
Anim-Baidoo I et al (2016) Giardia lamblia infections in children in Ghana. Pan Afr Med J 24:217
Ayalew D et al (2008) Cryptosporidium and Giardia infection and drinking water sources among children in Lege Dini. Ethiopia Trop Med Int Health 13(4):472–475
Becker SL et al (2015) Combined stool-based multiplex PCR and microscopy for enhanced pathogen detection in patients with persistent diarrhoea and asymptomatic controls from Cote d'Ivoire. Clin Microbiol Infect 21(6):591e1–591e59110
Belete YA, Kassa TY, Baye MF (2021) Prevalence of intestinal parasite infections and associated risk factors among patients of Jimma health center requested for stool examination, Jimma, Ethiopia. PLoS One 16(2):e0247063
Beyene G, Tasew H (2014) Prevalence of intestinal parasite, Shigella and Salmonella species among diarrheal children in Jimma health center, Jimma southwest Ethiopia: a cross sectional study. Ann Clin Microbiol Antimicrob 13:10
Birrie H, Erko B (2017) Giardiasis in Ethiopia. Ethiop J Heal Dev 9(2):80384077. https://api.semanticscholar.org/CorpusID:80384077
Breathnach AS, McHugh TD, Butcher PD (2010) Prevalence and clinical correlations of genetic subtypes of Giardia lamblia in an urban setting. Epidemiol Infect 138(10):1459–1467
Brunn A et al (2019) The influence of climate and livestock reservoirs on human cases of giardiasis. Ecohealth 16(1):116–127
Cacciò SM, Sprong H (2011) Epidemiology of giardiasis in humans. In: Luján HD, Svärd S (eds) Giardia: A Model Organism. Springer Vienna, Vienna, pp 17–28
Damitie M et al (2018) Molecular epidemiology of Giardia duodenalis infection in humans in Southern Ethiopia: a triosephosphate isomerase gene-targeted analysis. Infect Dis Poverty 7(1):17
de Lucio A et al (2016) Prevalence and genetic diversity of giardia duodenalis and cryptosporidium spp. among school children in a rural area of the Amhara region, north-west Ethiopia. PLoS One 11(7):e0159992
Einarsson E, Ma'ayeh S, Svard SG (2016) An up-date on Giardia and giardiasis. Curr Opin Microbiol 34:47–52
Fantinatti M et al (2016) Identification of Giardia lamblia Assemblage E in humans points to a new anthropozoonotic cycle. J Infect Dis 214(8):1256–1259
Feng Y, Xiao L (2011) Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev 24(1):110–140
Flecha MJ et al (2015) Detection and molecular characterisation of Giardia duodenalis, Cryptosporidium spp. and Entamoeba spp. among patients with gastrointestinal symptoms in Gambo Hospital, Oromia Region, southern Ethiopia. Tropical Med Int Health 20(9):1213–1222
GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963):117–171. https://doi.org/10.1016/S0140-6736(14)61682-2
Gelanew T et al (2007) Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop 102(2):92–99
Hajare ST, Chekol Y, Chauhan NM (2022) Assessment of prevalence of Giardia lamblia infection and its associated factors among government elementary school children from Sidama zone, SNNPR, Ethiopia. PLoS One 17(3):e0264812
Haque R et al (2005) Giardia assemblage A infection and diarrhea in Bangladesh. J Infect Dis 192(12):2171–2173
Haque R et al (2009) Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis 48(9):1191–1197
Heyworth MF (2016) Giardia duodenalis genetic assemblages and hosts. Parasite 23:13. https://doi.org/10.1051/parasite/2016013
Hijjawi N et al (2022) A review of the molecular epidemiology of Cryptosporidium spp. and Giardia duodenalis in the Middle East and North Africa (MENA) region. Infect Genet Evol 98:105212
Ignatius R et al (2012) High prevalence of Giardia duodenalis Assemblage B infection and association with underweight in Rwandan children. PLoS Negl Trop Dis 6(6):e1677
Johansen OH et al (2021) Performance and operational feasibility of two diagnostic tests for cryptosporidiosis in children (CRYPTO-POC): a clinical, prospective, diagnostic accuracy study. Lancet Infect Dis 21(5):722–730
Kabir F et al (2022) Impact of enteropathogens on faltering growth in a resource-limited setting. Front Nutr 9:1081833
Keselman A et al (2016) The microbiota contributes to CD8+ T cell activation and nutrient malabsorption following intestinal infection with Giardia duodenalis. Infect Immun 84(10):2853–2860
Kifleyohannes T et al (2022) Cryptosporidium and Giardia infections in humans in Tigray, Northern Ethiopia: an unexpectedly low occurrence of anthropozoonotic transmission. Acta Trop 231:106450
Leder K, Weller DPF (2020) Giardiasis: epidemiology, clinical manifestations, and diagnosis. UpToDate. [Accessed on: 15 March 2019]
Leung AKC et al (2019) Giardiasis: an overview. Recent Patents Inflamm Allergy Drug Discov 13(2):134–143
Mahdavi F et al (2022) Global epidemiology of Giardia duodenalis infection in cancer patients: a systematic review and meta-analysis. Int Health 14(1):5–17
Mbae C et al (2016) Molecular characterization of giardia duodenalis in children in Kenya. BMC Infect Dis 16:135
Mengistu A, Gebre-Selassie S, Kassa T (2007) Prevalence of intestinal parasitic infections among urban dwellers in southwest Ethiopia. Ethiop J Health Dev 21(1):12–17
Messa A Jr et al (2021) Molecular diversity of Giardia duodenalis in children under 5 years from the Manhica district, Southern Mozambique enrolled in a matched case-control study on the aetiology of diarrhoea. PLoS Negl Trop Dis 15(1):e0008987
Modi P et al (2015) Midupper arm circumference outperforms weight-based measures of nutritional status in children with diarrhea. J Nutr 145(7):1582–1587
Molina N et al (2011) High prevalences of infection with Giardia intestinalis genotype B among children in urban and rural areas of Argentina. Ann Trop Med Parasitol 105(4):299–309
Muhsen K, Levine MM (2012) A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clin Infect Dis 55:S271–S293
Mwangome MK et al (2011) Are diagnostic criteria for acute malnutrition affected by hydration status in hospitalized children? A repeated measures study. Nutr J 10:92
Nundy S et al (2011) Wealth and its associations with enteric parasitic infections in a low-income community in Peru: use of principal component analysis. Am J Trop Med Hyg 84(1):38–42
Pipikova J et al (2020) First report on Giardia duodenalis assemblage F in Slovakian children living in poor environmental conditions. J Microbiol Immunol Infect 53(1):148–156
Read C et al (2002) Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol 32(2):229–231
Rogawski ET et al (2017) Determinants and impact of giardia infection in the first 2 years of life in the MAL-ED birth cohort. J Pediatric Infect Dis Soc 6(2):153–160
Samie A et al (2020) Prevalence and genetic characterization of Giardia lamblia in relation to diarrhea in Limpopo and Gauteng provinces. South Africa Parasite Epidemiol Contr 9:e00140
Siwila J et al (2011) Seasonal prevalence and incidence of Cryptosporidium spp. and Giardia duodenalis and associated diarrhoea in children attending pre-school in Kafue, Zambia. Trans R Soc Trop Med Hyg 105(2):102–108
Soliman RH, Fuentes I, Rubio JM (2011) Identification of a novel Assemblage B subgenotype and a zoonotic Assemblage C in human isolates of Giardia intestinalis in Egypt. Parasitol Int 60(4):507–511
Stensvold CR, Nielsen HV (2012) Comparison of microscopy and PCR for detection of intestinal parasites in Danish patients supports an incentive for molecular screening platforms. J Clin Microbiol 50(2):540–541
Tamura K et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tellevik MG et al (2015) Prevalence of Cryptosporidium parvum/hominis, Entamoeba histolytica and Giardia lamblia among young children with and without diarrhea in Dar es Salaam, Tanzania. PLoS Negl Trop Dis 9(10):e0004125
Tigabu E, Petros B, Endeshaw T (2010) Prevalence of giardiasis and cryptosporidiosis among children in relation to water sources in selected village of Pawi Special District in Benishangul-Gumuz Region, northwestern Ethiopia. Ethiop J Health Dev 24(3). https://doi.org/10.4314/ejhd.v24i3.68387
Van Lith L et al (2015) A real-time assemblage-specific PCR assay for the detection of Giardia duodenalis assemblages A, B and E in fecal samples. Vet Parasitol 211(1-2):28–34
Wegayehu T, Adamu H, Petros B (2013) Prevalence of Giardia duodenalis and Cryptosporidium species infections among children and cattle in North Shewa Zone Ethiopia. BMC Infect Dis 13:419
Wegayehu T et al (2016) Multilocus genotyping of Giardia duodenalis isolates from children in Oromia Special Zone, central Ethiopia. BMC Microbiol 16:89
WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards: Length/height-for-age, weightfor-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization, p 312
Acknowledgements
We thank the clinical and laboratory staff in Jimma Medical Centre, Jimma, Ethiopia; Serbo Health Centre, Serbo, Ethiopia; and all children who participated, and their caregivers.
Funding
Open access funding provided by University of Bergen (incl Haukeland University Hospital) This study was funded by the Norwegian Research Council GLOBVAC fund (grant 255571), Bill and Melinda Gates Foundation (grant OPP1153139), Norwegian association for medical microbiology, University of Bergen, Vestfold Hospital Trust, JPIAMR (grant number NFR333432), and Trond Mohn Foundation (grant number TMS2020TMT11).
Author information
Authors and Affiliations
Consortia
Contributions
Øystein H. Johansen, Kurt Hanevik, and Nina Langeland designed the study and obtained funding. Sabrina John Moyo, Alemseged Abdissa, and Zeleke Mekonnen contributed to the study design. Alemseged Abdissa oversaw local data collection and data management. Øystein H. Johansen, Yonas Alemu, Zeleke Mekonnen, and Bizuwarek Sharew supervised the clinical and laboratory staff. Yonas Alemu did the real-time PCR and Bizuwarek Sharew did IFAT. Sabrina John Moyo did the statistical analysis. Sabrina John Moyo and Yonas Alemu wrote the first draft of the manuscript. All authors read, contributed to the editing of the manuscript, and agreed to be accountable for all aspects of the approved final version manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was obtained for the project from Jimma University IRB (Reference: RPGC/610/2016), the Ethiopian National Research Ethics Review Committee (Reference: JU JURPGD/839/2017), and the Regional Committee for Medical and Health Research Ethics of Western Norway (Reference: 2016/1096).
Consent to participate
Written informed consent to participate and publish results was obtained from parents/caretaker of the children. Results from the laboratory analysis were communicated to the responsible pediatrician for early initiation of treatment.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Yaoyu Feng
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alemu, Y., Abdissa, A., Mekonnen, Z. et al. Prevalence and assemblage of Giardia duodenalis in a case-control study of children under 5 years from Jimma, Southwest Ethiopia. Parasitol Res 123, 38 (2024). https://doi.org/10.1007/s00436-023-08029-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-023-08029-5